Development of Metal Cluster Chemistry Using π-Metal Orbital Interaction
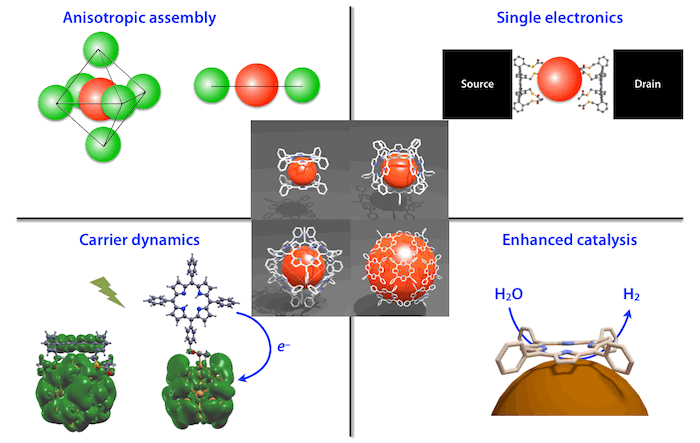
Porphyrin-Au Nanoparticles & Clusters
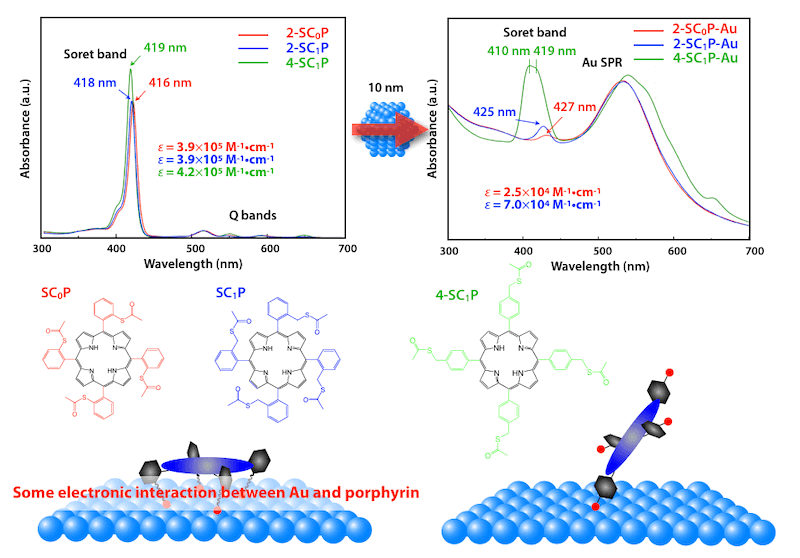
Gold(0) Porphyrins on Gold Nanoparticles
(Angew. Chem. Int. Ed. 2008, 47, 307.)
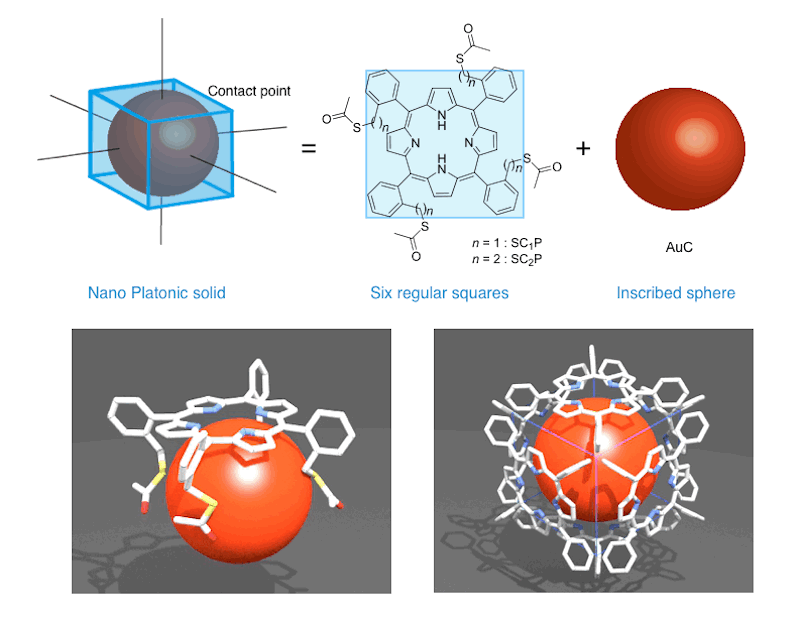
Platonic Hexahedron Composed of Six Organic Faces with an Inscribed Au Cluster
(J. Am. Chem. Soc. 2012, 134, 816.)
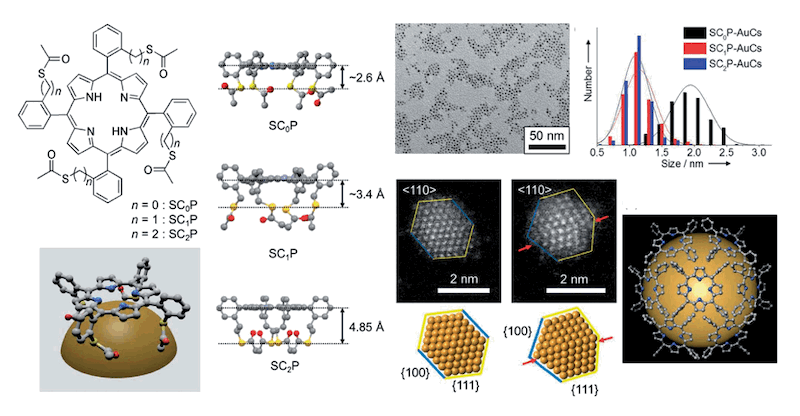
Strongest π-Metal Orbital Coupling in a Porphyrin/Gold Cluster System
(Chem. Sci. 2014, 5, 2007.)
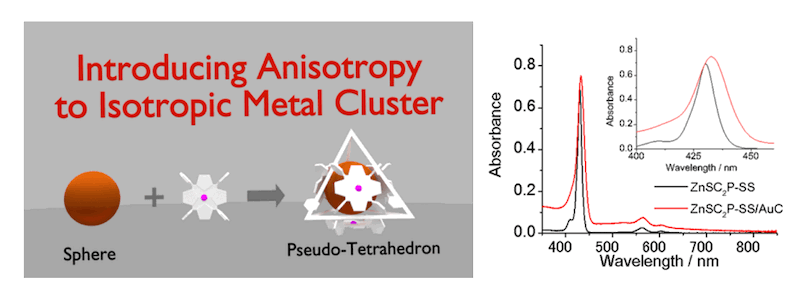
Porphyrin Derivative-Protected Gold Cluster with a Pseudotetrahedral Shape
(J. Phys. Chem. C 2017, 121, 10760.)
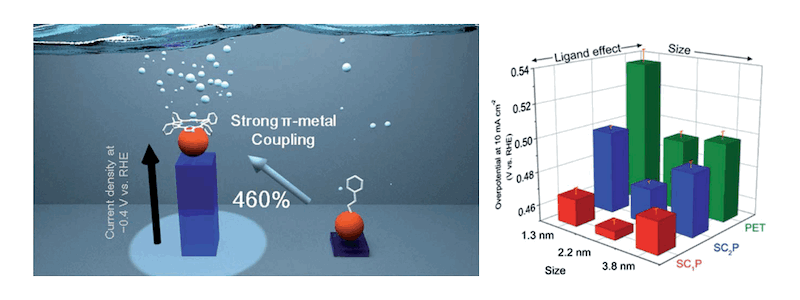
Ligand Effect on the Catalytic Activity of Gold Clusters in the Electrochemical Hydrogen Evolution Reaction
(Chem. Sci. 2018, 9, 261.)
Impact of Orbital Hybridization at Molecular-Metal Interface on Carrier Dynamics
(J. Phys. Chem. C 2019, 123, 25877. (Cover Picture))