Synthesis of Unprecedented Nanoparticles by Element Substitution Science and Development of Their Novel Functions
Noble metal nanoparticles (NPs) are excellent functional materials based on the band structures that the d band centers are a few eV lower than the Fermi levels. Therefore, it seems difficult to replace the noble metal NPs with other NPs. In order to create novel nanomaterials that surpass the conventional performance of noble metals, the ground-state electronic structures (band structure, Fermi level, defect level) of 3d and 4d transition metals should be largely modulated by a simple method. Because of a large specific surface area of nanoparticles, the element substitution reaction easily progresses, and the ground-state electronic structure of 3d and 4d transition metals can be largely modulated. In 3d and 4d metal nanoparticles, the electronic structure may be modulated by introducing p-block elements or alloying with metals with low redox potentials (galvanic substitution). In addition, in heavy-doped semiconductor nanoparticles composed of d-block metals, the crystal structure and electronic structure can be partially modulated by element substitution (ion exchange) to fabricate heterostructured nanoparticles for near-infrared light energy conversion.
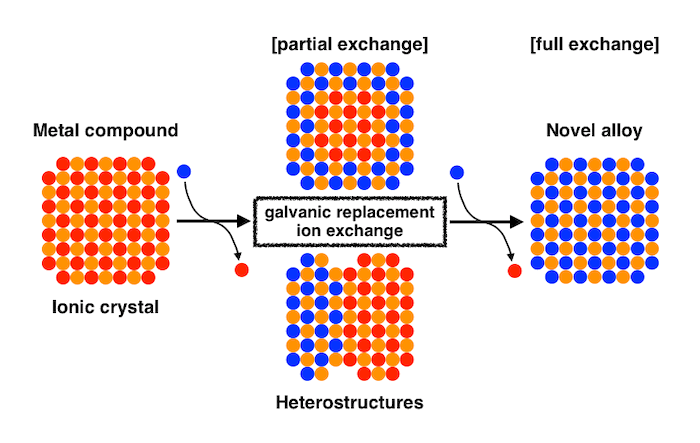
Formation of Novel Structures by Ion Exchange Reactions of Ionic Crystal Nanoparticles
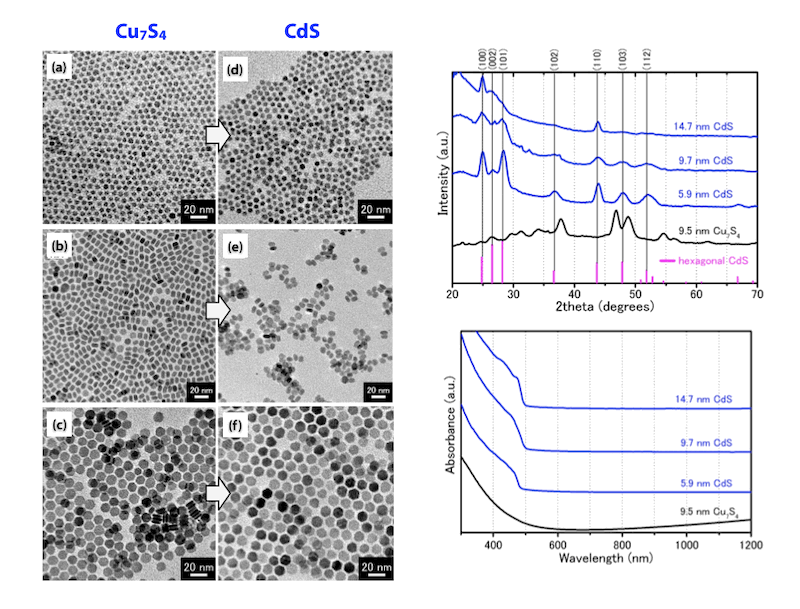
Large-Scale Synthesis of High-Quality Metal Sulfide Semiconductor Quantum Dots with Tunable Surface-Plasmon Resonance Frequencies
(Chem. Eur. J. 2012, 18, 9230. (Frontispiece))
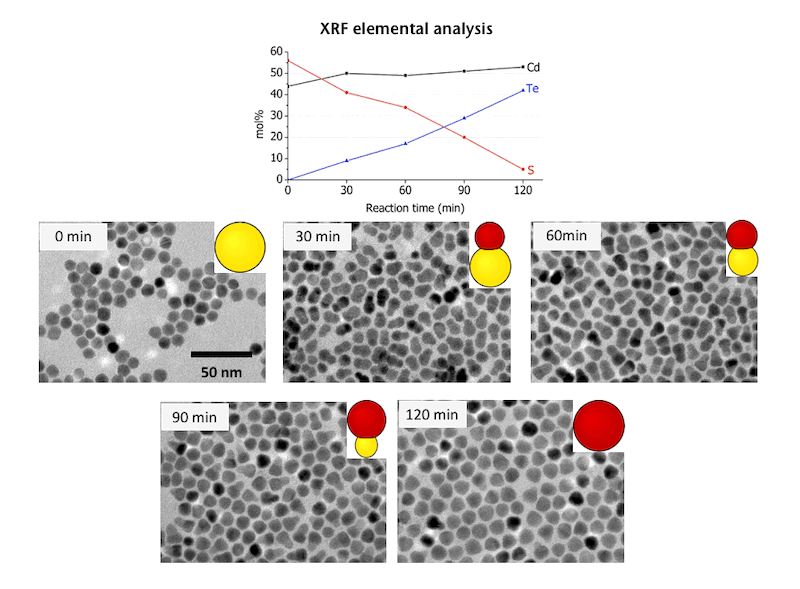
Formation of Hexagonal CdS-Cubic CdTe Heterostructured Nanoparticles & Cubic CdTe Nanoparticles by Anion Exchange of Hexagonal CdS Nanoparticles
(J. Am. Chem. Soc. 2011, 133, 17598; Chem. Sci. 2014, 5, 3831;
J. Phys. Chem. Lett. 2014, 5, 2951.)
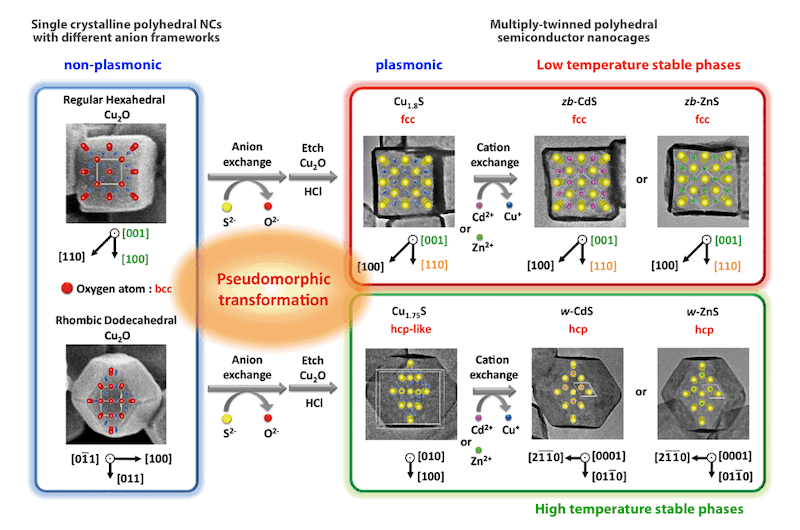
Formation of Pseudomorphic Nanocages from Cu2Oナノ粒子 Nanocrystals through Anion Exchange Reactions
(Science 2016, 351, 1306.)



