- バックアップ一覧
- 差分 を表示
- 現在との差分 を表示
- ソース を表示
- research へ行く。
- 1 (2017-07-25 (火) 16:31:08)
- 2 (2017-07-25 (火) 19:32:35)
- 3 (2017-07-25 (火) 19:50:38)
- 4 (2017-07-26 (水) 10:50:59)
- 5 (2017-07-26 (水) 15:52:26)
- 6 (2017-07-28 (金) 11:40:13)
- 7 (2017-07-28 (金) 19:33:59)
- 8 (2017-08-02 (水) 11:15:07)
- 9 (2017-08-02 (水) 20:12:01)
- 10 (2017-08-03 (木) 17:52:17)
- 11 (2017-08-07 (月) 12:10:16)
- 12 (2017-09-22 (金) 00:52:16)
- 13 (2017-11-04 (土) 07:17:32)
- 14 (2017-11-13 (月) 18:36:42)
- 15 (2018-06-05 (火) 00:31:50)
- 16 (2018-06-08 (金) 19:48:42)
- 17 (2018-06-08 (金) 11:01:31)
- 18 (2019-05-11 (土) 16:07:01)
- 19 (2019-05-20 (月) 10:33:42)
- 20 (2019-09-27 (金) 18:58:26)
- 21 (2020-01-14 (火) 22:39:24)
- 22 (2020-05-13 (水) 20:59:03)
- 23 (2020-05-16 (土) 00:11:32)
- 24 (2020-06-20 (土) 00:11:28)
- 25 (2020-10-05 (月) 19:36:57)
- 26 (2020-10-05 (月) 21:04:21)
- 27 (2020-10-12 (月) 22:40:50)
- 28 (2021-01-19 (火) 23:09:54)
- 29 (2021-02-13 (土) 00:43:20)
- 30 (2021-02-16 (火) 18:10:50)
- 31 (2021-09-16 (木) 23:10:09)
- 32 (2021-09-16 (木) 23:45:31)
- 33 (2021-09-27 (月) 21:02:32)
- 34 (2021-09-28 (火) 23:39:36)
- 35 (2021-10-12 (火) 20:40:04)
- 36 (2021-10-13 (水) 00:13:03)
- 37 (2022-04-12 (火) 19:51:00)
- 38 (2022-04-12 (火) 22:28:12)
- 39 (2022-05-09 (月) 22:06:00)
- 40 (2022-05-09 (月) 22:06:46)
- 41 (2022-09-13 (火) 09:37:47)
- 42 (2022-09-13 (火) 09:44:46)
- 43 (2022-09-22 (木) 09:01:29)
- 44 (2022-10-13 (木) 12:32:11)
- 45 (2022-10-31 (月) 09:32:44)
- 46 (2022-11-04 (金) 09:57:16)
- 47 (2023-09-19 (火) 09:53:01)
- 48 (2023-09-19 (火) 12:18:04)
- 49 (2023-10-02 (月) 09:31:55)
- 50 (2023-11-22 (水) 15:36:45)
 | Research |

Create New World of Bioactive Synthetic Molecules
New ways to use, New Shapes, New sizes. †
As biological processes all stem from chemical events, it should be possible to understand or manipulate biological events by using chemistry. As chemical biologists our challenge is to discover or design unique organic molecules, 'super tools' that modulate fundamental processes in human cells. Using these 21st century super tools we can explore complex cellular events. Our job is creation: designing a new world of bioactive synthetic molecules, with unique and novel uses, forms and sizes. The future for small-molecule applications is open before us, in a range of fields, including future drug discovery and cell therapy. Our 21st century technology and skills will revolutionize the fight against the most difficult diseases and medical challenges of the age.
ケミカルバイオロジー~化学を起点とした生物学 †
ケミカルバイオロジーとは、化学を起点とした生物学です。生命の営みはせんじつめれば化学反応でできています。逆に化学を使って生命現象を理解したり、操ることができるはず。私たちの研究室では、生き物やヒト細胞にユニークな効果を及ぼす独自な有機化合物を見つける、もしくはデザインし、それらを道具として生命現象を探究・操作してきました。生物の仕組みは複雑ですが、有機化合物を起爆剤として用いることで、新たな切り口で生物を研究したり操作することができます。 私たちの大きな研究目標は、生理活性合成化合物の新しい世界を切り開くことです。生理活性化合物の新しい利用法、新しいカタチをした生理活性化合物、新しいサイズの生理活性化合物など、アイデアを与えることができればと思うのです。こういったアイデアは、未来の創薬の考え方や化合物の細胞治療への利用などに結びつくはずです。
Selected Publications †
Controlled lipid β-oxidation and carnitine biosynthesis by a vitamin D metabolite. †
Mendoza, A., Takemoto, Y., Cruzado, K., Masoud, S., Nagata, A., Tantipanjaporn, A., Okuda, S., Kawagoe, F., Sakamoto, R., Odagi, M., Mototani, S., Togashi, M., Kawatani, M., Aono, H., Osada, H., Nakagawa, H., Higashi, T., Kittaka, A., Nagasawa, K., Uesugi, M. †
Cell Chemical Biology (In press) (2021)
| =Lactone-vitamin D3 is a major metabolite of vitamin D3, a lipophilic vitamin biosynthesized in numerous life forms by sunlight exposure. Although lactone-vitamin D3 was discovered 40 years ago, its biological role remains largely unknown. Chemical biological analysis of its photoaffinity probe identified the hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha (HADHA), a mitochondrial enzyme that catalyzes β-oxidation of long-chain fatty acids, as its selective binding protein. Intriguingly, the interaction of lactone-vitamin D3 with HADHA does not affect the HADHA enzymatic activity but instead limits biosynthesis of carnitine, an endogenous metabolite required for the transport of fatty acids into the mitochondria for β-oxidation. Lactone-vitamin D3 dissociates the protein-protein interaction of HADHA with trimethyllysine dioxygenase (TMLD), thereby impairing the TMLD enzyme activity essential in carnitine biosynthesis. These findings suggest a heretofore undescribed role of lactone-vitamin D3 in lipid β-oxidation and carnitine biosynthesis, and possibly in sunlight-dependent shifts of lipid metabolism in animals. | 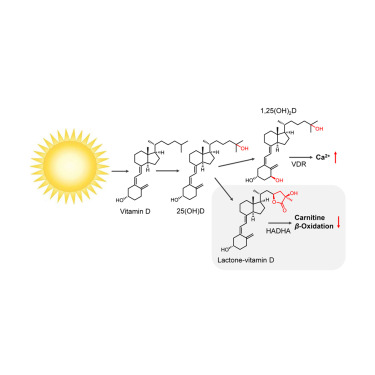 |
Chemical Genetics Reveals a Role of Squalene Synthase in TGFβ Signaling and Cardiomyogenesis †
Takemoto, Y., Kadota, S., Minami, I., Otsuka, S., Okuda, S., Abo, M., Punzalan, L., Shen, Y., Shiba, Y., Uesugi, M. †
Angew. Chem. Int. Ed. (In press) (2021)
| =KY02111 is a widely used small molecule that boosts cardiomyogenesis of the mesoderm cells derived from pluripotent stem cells, yet its molecular mechanism of action remains elusive. The present study resolves the initially perplexing effects of KY02111 on Wnt signaling and subsequently identifies squalene synthase (SQS) as a molecular target of KY02111 and its optimized version KY-I. By disrupting the interaction of SQS with cardiac ER-membrane protein TMEM43, KY02111 impairs TGFβ signaling, but not Wnt signaling, and thereby recapitulates the clinical mutation of TMEM43 that causes arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited heart disease that involves a substitution of myocardium with fatty tissue. These findings reveal a heretofore undescribed role of SQS in TGFβ signaling and cardiomyogenesis. KY02111 may find its use in ARVC modeling as well as serve as a chemical tool for studying TGFβ/SMAD signaling. | 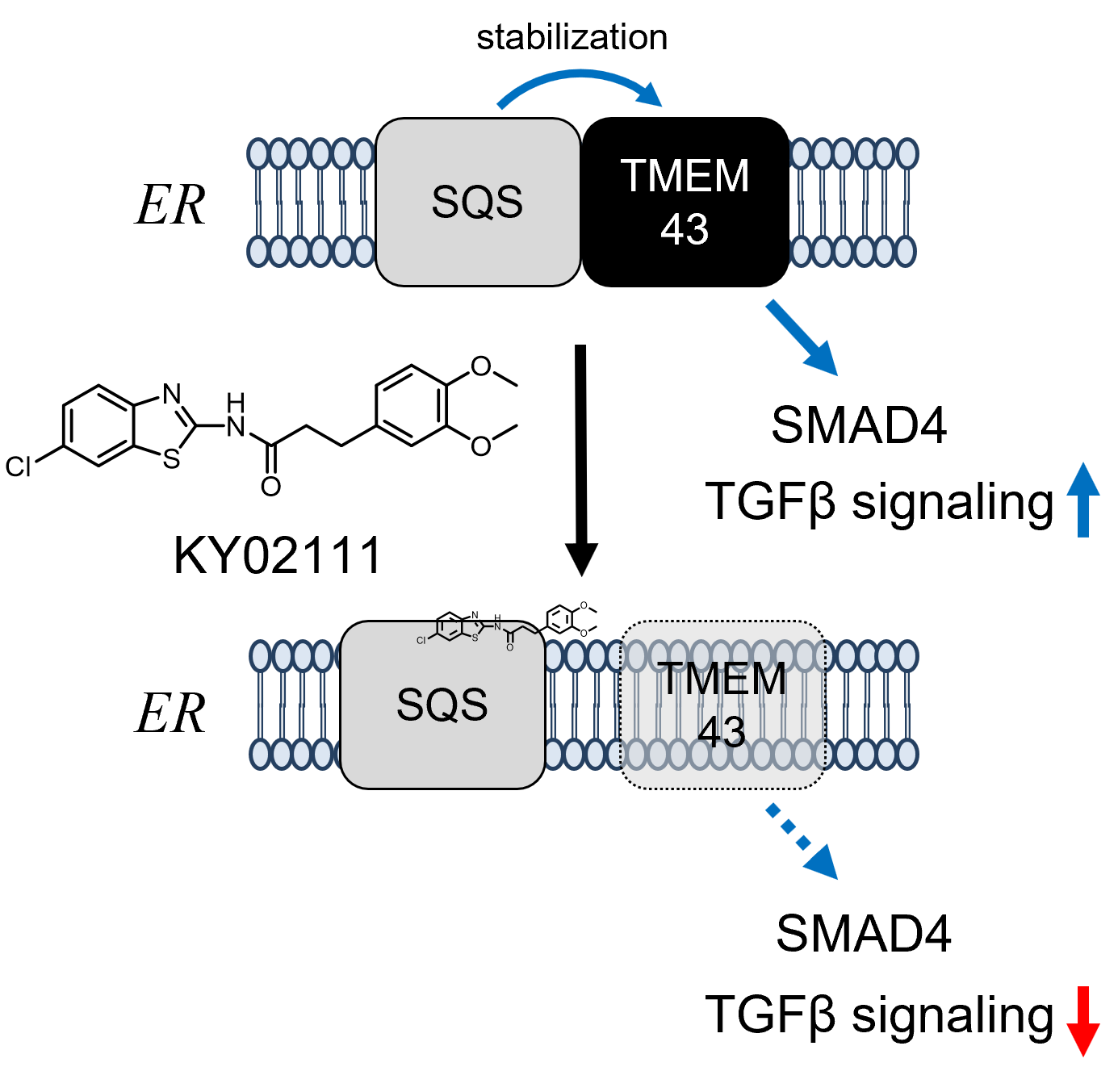 |
Non-genetic cell-surface modification with a self-assembling molecular glue. †
Hakariya, H., Takashima, I., Takemoto, M., Noda, N., Sato, S., Uesugi, M. †
Chem. Comm. 57(12), 1470-1473 (2021)
| =This report describes the development of a non-genetic cell-surface modification method, in which a self-assembling small molecule is combined with Halo-tag proteins. Cell-surface functionalization with cancer-linked extracellular proteins led to enhanced cell motility, angiogenesis, and immune shielding of the cells, paving the way for translational opportunities for cell therapy. | 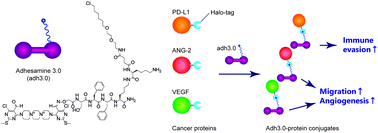 |
Discovery of Self‐Assembling Small Molecules as Vaccine Adjuvants. †
Jin, S., Vu, H., Hioki, K., Noda, N., Yoshida, H., Shimane, T., Ishizuka, S., Takashima, I., Mizuhata, Y., Pe, K., Ogawa, T., Nishimura, N., Packwood, D., Tokitoh, N., Kurata, H., Yamasaki, S., Ishii, K., Uesugi, M. †
Angew. Chem. Int. Ed. 60(2), 961-969 (2021)
| =Immune potentiators, termed adjuvant, trigger early innate immune responses to ensure the generation of robust and long‐lasting adaptive immune responses of vaccines. Here we present study that takes advantage of a self‐assembling small molecule library for the development of a novel vaccine adjuvant. Cell‐based screening of the library and subsequent structural optimization led to the discovery of a simple, chemically tractable deoxycholate derivative (molecule 6 , also named cholicamide) whose well‐defined nano‐assembly potently elicits innate immune responses in macrophages and dendritic cells. Functional and mechanistic analyses indicate that the virus‐like assembly is engulfed inside cells and stimulates the innate immune response through toll‐like receptor 7 (TLR7), an endosomal TLR that detects single‐stranded viral RNA. As an influenza vaccine adjuvant in mice, molecule 6 was as potent as Alum, a clinically used adjuvant. The studies described here pave the way for a new approach to discovering and designing self‐assembling small‐molecule adjuvants against pathogens, including emerging viruses. | 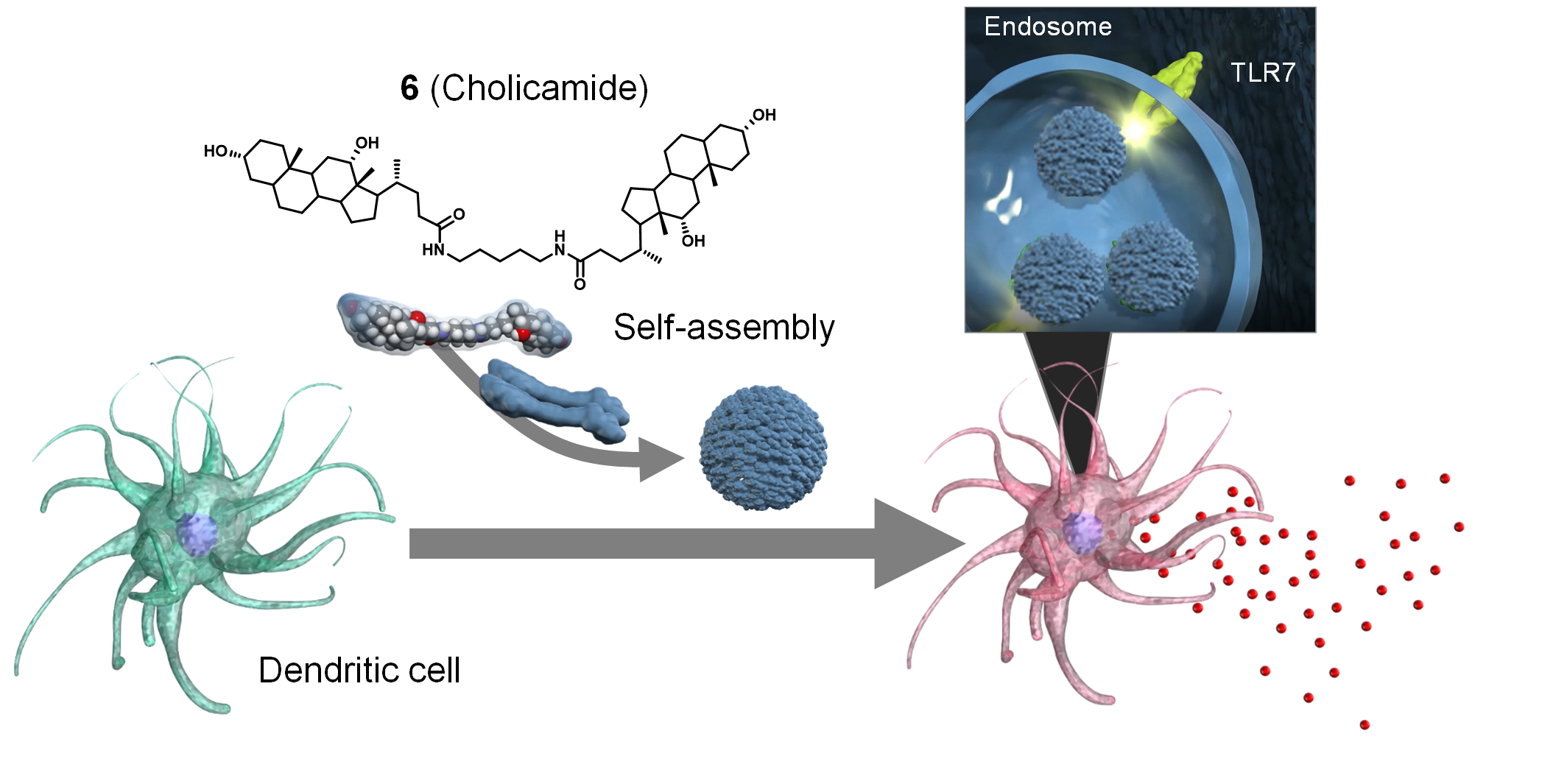 |
Chemoproteomic Profiling of a Pharmacophore-Focused Chemical Library. †
Punzalan, L., Jiang, L., Mao, D., Mahapatra, A., Sato, S., Takemoto, Y., Tsujimura, M., Kusamori, K., Nishikawa, M., Zhou, L., Uesugi, M. †
Cell Chemical Biology 27(6), 708–718 (2020)
| =Pharmacophore-focused chemical libraries are continuously being created in drug discovery programs, yet screening assays to maximize the usage of such libraries are not fully explored. Here, we report a chemical proteomics approach to reutilizing a focused chemical library of 1,800 indole-containing molecules for discovering uncharacterized ligand-protein pairs. Gel-based protein profiling of the library using a photo-affinity indole probe 1 enabled us to find new ligands for glyoxalase 1 (Glo1), an enzyme involved in the detoxification of methylglyoxal. Structure optimization of the ligands yielded an inhibitor for Glo1 ( 9). Molecule 9 increased the cellular methylglyoxal levels in human cells and suppressed the osteoclast formation of mouse bone marrow-derived macrophages. X-ray structure analyses revealed that the molecule lies at a site abutting the substrate binding site, which is consistent with the enzyme kinetic profile of 9. Overall, this study exemplifies how chemical proteomics can be used to exploit existing focused chemical libraries. | 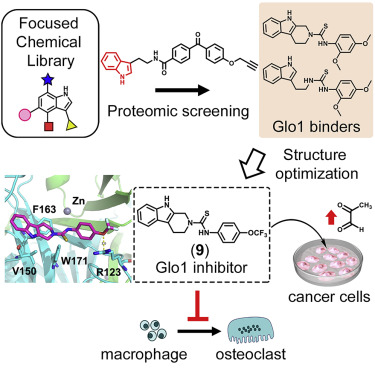 |
Discovery of a Small-Molecule-Dependent Photolytic Peptide. †
Takemoto, Y., Mao, Di., Punzalan, L., Götze, S., Sato, S., Uesugi, M. †
J. Am. Chem. Soc. 142(3), 1142-1146 (2020)
| =We accidentally found that YM-53601, a known small-molecule inhibitor of squalene synthase (SQS), selectively depletes SQS from mammalian cells upon UV irradiation. Further analyses indicated that the photodepletion of SQS requires its short peptide segment located at the COOH terminus. Remarkably, when the 27 amino acid peptide was fused to green fluorescent protein or unrelated proteins at either the NH2 or COOH terminus, such fusion proteins were selectively depleted when the cells were treated with both YM-53601 and UV exposure. Product analysis and electron spin resonance experiments suggested that the UV irradiation promotes homolytic C–O bond cleavage of the aryl ether group in YM-53601. It is likely that the radical species generated from UV-activated YM-53601 abstract hydrogen atoms from the SQS peptide, leading to the photolysis of the entire protein. The pair of the SQS peptide and YM-53601 discovered in the present study paves the way for the design of a new small-molecule-controlled optogenetic tool. | 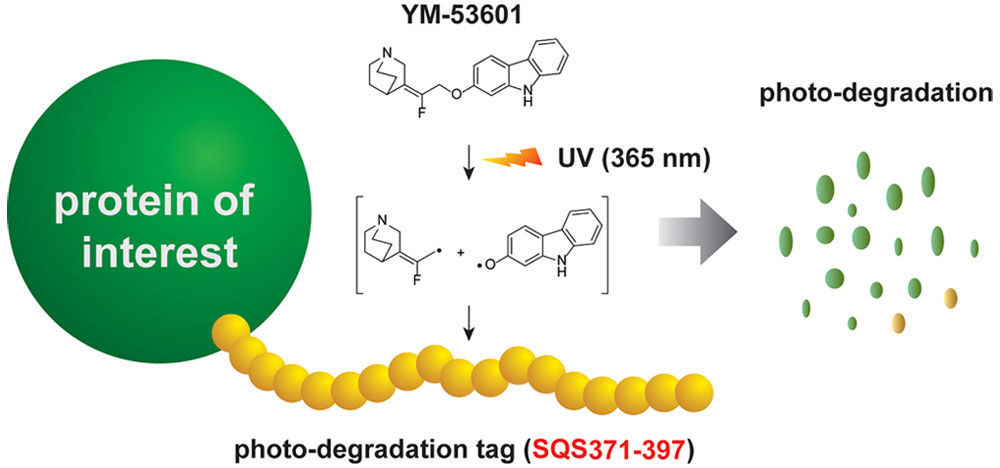 |
Synthetic Chemical Probes That Dissect Vitamin D Activities. †
Nagata, A., Akagi, Y., Asano, L., Kotake, K., Kawagoe, F., Mendoza, A., Masoud, S., Usuda, K., Yasui, K., Takemoto, Y., Kittaka, A., Nagasawa, K., Uesugi, M. †
ACS Chem. Biol. 14(12), 2851–2858 (2019)
| =Vitamin D3 metabolites are capable of controlling gene expression in mammalian cells through two independent pathways: vitamin D receptor (VDR) and sterol regulatory element-binding protein (SREBP) pathways. In the present study, we dissect the complex biological activity of vitamin D by designing synthetic vitamin D3 analogs specific for VDR or SREBP pathway, i.e., a VDR activator that lacks SREBP inhibitory activity, or an SREBP inhibitor devoid of VDR activity. These synthetic vitamin D probes permitted identification of one of the vitamin D-responsive genes, Soat1, as an SREBP-suppressed gene. The chemical probes developed in the present study may prove useful in dissecting the intricate interplay of vitamin D actions, thereby providing insights into how vitamin D target genes are regulated. | 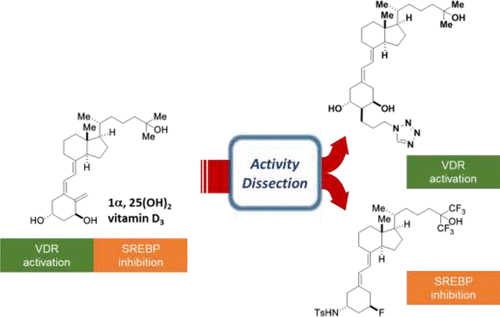 |
Nutrient-Based Chemical Library as a Source of Energy Metabolism Modulators. †
Furuta,T., Mizukami, Y., Asano, L., Kotake, K., Ziegler, S., Yoshida, H., Watanabe, M., Sato, S., Waldmann, H., Nishikawa, M., Uesugi, M. †
ACS Chem. Biol. 14(9), 1860-1865 (2019)
| =Covalent conjugates of multiple nutrients often exhibit greater biological activities than each individual nutrient and more predictable safety profiles than completely unnatural chemical entities. Here, we report the construction and application of a focused chemical library of 308 covalent conjugates of a variety of small-molecule nutrients. Screening of the library with a reporter gene of sterol regulatory element-binding protein (SREBP), a master regulator of mammalian lipogenesis, led to the discovery of a conjugate of docosahexaenoic acid (DHA), glucosamine, and amino acids as an inhibitor of SREBP (molecule 1, DHG). Mechanistic analyses indicate that molecule 1 impairs the SREBP activity by inhibiting glucose transporters and thereby activating AMP-activated protein kinase (AMPK). Oral administration of molecule 1 suppressed the intestinal absorption of glucose in mice. These results suggest that such synthetic libraries of nutrient conjugates serve as a source of novel chemical tools and pharmaceutical seeds that modulate energy metabolism. | 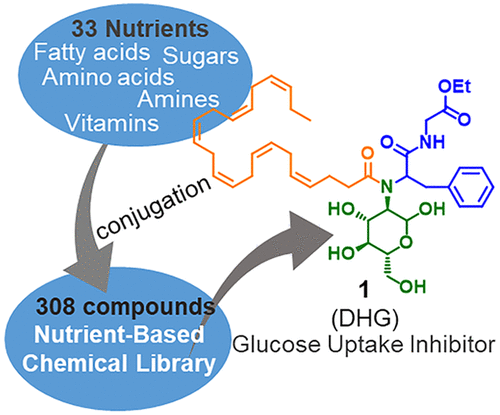 |
Multifunctionalization of cells with a self-assembling molecule to enhance cell engraftment. †
Takashima, I., Kusamori, K., Hakariya, H., Takashima, M., Vu, TH., Mizukami, Y., Noda, N., Takayama, Y., Katsuda, Y., Sato, S., Takakura, Y., Nishikawa, M., Uesugi, M. †
ACS Chem. Biol., 14(4), 775–783 (2019).
| =Cell-based therapy is a promising approach to restoring lost functions to compromised organs. However, the issue of inefficient cell engraftment remains to be resolved. Herein, we take a chemical approach to facilitate cell engraftment by using self-assembling molecules which modify two cellular traits: cell survival and invasiveness. In this system, the self-assembling molecule induces syndecan-4 clusters on the cellular surface, leading to enhanced cell viability. Further integration with Halo-tag technology provided this self-assembly structure with matrix metalloproteinase-2 to functionalize cells with cell-invasion activity. In vivo experiments showed that the pretreated cells were able to survive injection and then penetrate and engraft into the host tissue, demonstrating that the system enhances cell engraftment. Therefore, cell-surface modification via an alliance between self-assembling molecules and ligation technologies may prove to be a promising method for cell engraftment. | 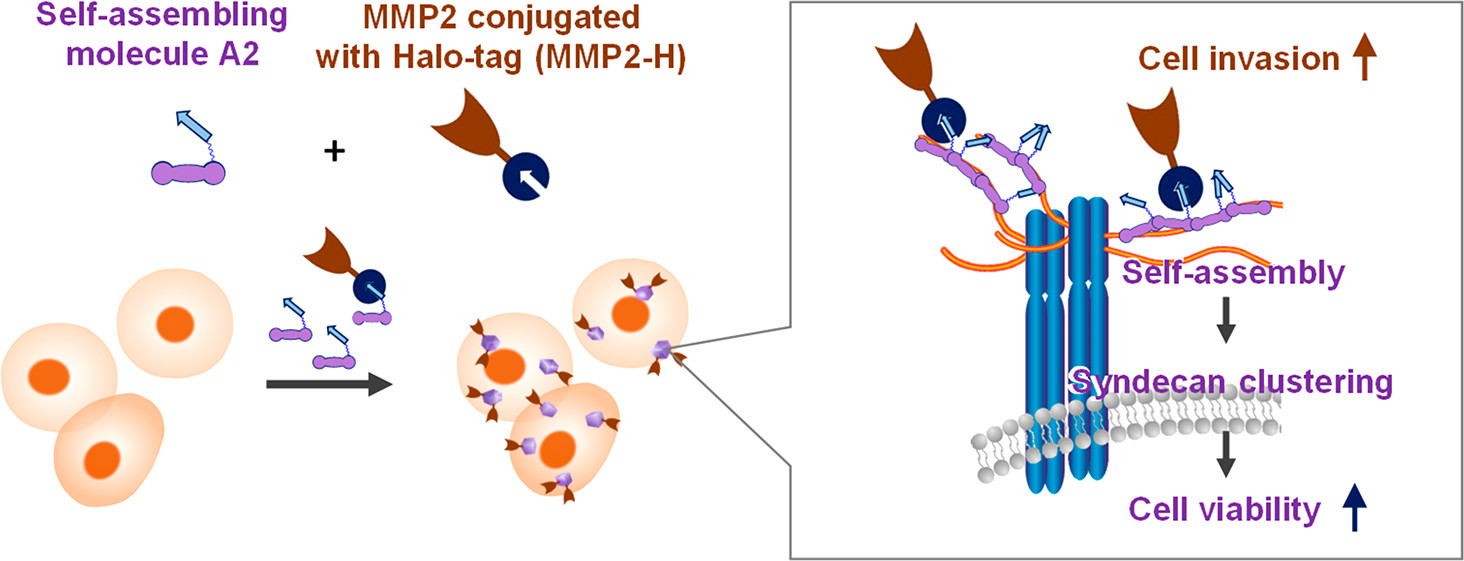 |
Small-molecule screening yields a compound that inhibits the cancer-associated transcription factor Hes1 via the PHB2 chaperone. †
Perron, A., Nishikawa, Y., Iwata, J., Shimojo, H., Takaya, J., Kobayashi, K., Imayoshi, I., Mbenza, N. M,, Takenoya, M., Kageyama, R., Kodama, Y., Uesugi, M. †
J. Biol. Chem. 293(21), 8285-8294 (2018).
| =The transcription factor Hes family basic helix-loop-helix transcription factor 1 (Hes1) is a downstream effector of Notch signaling. In the present study, we report the discovery of small organic molecules (JI051 and JI130) that impair the ability of Hes1 to repress transcription. We found that JI051 interacts with prohibitin2(PHB2), a cancer-associated protein chaperone. JI051 dose-dependently reduced cell growth of the human pancreatic cancer cell line MIA PaCa-2, and JI130 treatment significantly reduced tumor volume in a murine pancreatic tumor xenograft model. These results suggest a previously unrecognized role for PHB2 in the regulation of Hes1 and may inform potential strategies for managing pancreatic cancer. | ![zbc0181885870001[1].jpg zbc0181885870001[1].jpg](https://www.scl.kyoto-u.ac.jp:443/~uesugi/ja/index.php?plugin=ref&page=research&src=zbc0181885870001%5B1%5D.jpg) |
Chemical decontamination of iPS cell-derived neural cell mixtures. †
Mao, D., Chung, X. K. W., Andoh-Noda, T., Qin, Y., Sato, S., Takemoto, Y., Akamatsu, W., Okano, H., Uesugi, M. †
Chem. Commun. 54, 1355-1358 (2018).
| =This report describes the design and evaluation of phosphorylated 7-ethyl-10-hydroxycamptothecin (SN38-P), which selectively eliminates tumor-forming proliferative stem cells, including human induced pluripotent stem cells (hiPSCs) and neural stem cells, from iPSC-derived neural cell mixtures. Results of the present study demonstrate that simple phosphorylation of an anticancer drug can provide a safe, cost-effective, and chemically-defined tool for decontaminating hiPSC-derived neuron. | 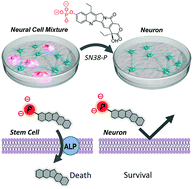 |
Vitamin D metabolite, 25-Hydroxyvitamin D, regulates lipid metabolism by inducing degradation of SREBP/SCAP. †
Asano, L., Watanabe, M., Ryoden, Y., Usuda, K., Yamaguchi, T., Khambu, B., Takashima, M., Sato, S., Sakai, J., Nagasawa, K., Uesugi, M. †
Cell Chem Biol. 24, 207-217 (2017).
| =Sterol regulatory element-binding proteins (SREBPs) are transcription factors that control lipid homeostasis. SREBP activation is regulated by a negative feedback loop in which sterols bind to SREBP cleavage-activating protein (SCAP), an escort protein essential for SREBP activation, or to insulin-induced genes (Insigs) (endoplasmic reticulum [ER] anchor proteins), sequestering the SREBP-SCAP-Insig complex in the ER. We screened a chemical library of endogenous molecules and identified 25-hydroxyvitamin D (25OHD) as an inhibitor of SREBP activation. Unlike sterols and other SREBP inhibitors, 25OHD impairs SREBP activation by inducing proteolytic processing and ubiquitin-mediated degradation of SCAP, thereby decreasing SREBP levels independently of the vitamin D receptor. The present results suggest a previously unrecognized molecular mechanism of vitamin D-mediated lipid control that might be useful in the treatment of metabolic diseases. | 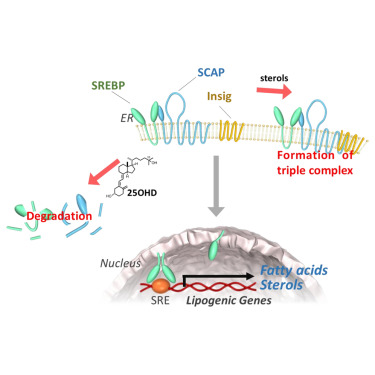 |
A synthetic hybrid molecule for the selective removal of human pluripotent stem cells from cell mixtures. †
Mao, D., Ando, S., Sato, S., Qin, Y., Hirata, N., Katsuda, Y., Kawase, E., Kuo, T.F., Minami, I., Shiba, Y., Ueda, K., Nakatsuji, N., Uesugi, M. †
Angew. Chem. Int. Ed. 56, 1765-1770 (2017).
| =A major hurdle in stem cell therapy is the tumorigenic risk of residual undifferentiated stem cells. This report describes the design and evaluation of synthetic hybrid molecules that efficiently reduce the number of human induced pluripotent stem cells (hiPSCs) in cell mixtures. The design takes advantage of Kyoto probe 1 (KP-1), a fluorescent chemical probe for hiPSCs, and clinically used anticancer drugs. Among the KP-1–drug conjugates we synthesized, we found an exceptionally selective, chemically tractable molecule that induced the death of hiPSCs. Mechanistic analysis suggested that the high selectivity originates from the synergistic combination of transporter-mediated efflux and the cytotoxicity mode of action. The present study offers a chemical and mechanistic rationale for designing selective, safe, and simple reagents for the preparation of non-tumorigenic clinical samples. | 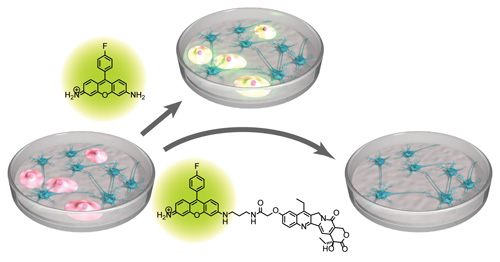 |
A small molecule that represses translation of G-quadruplex-containing mRNA. †
Katsuda, Y., Sato, S., Asano, L., Morimura, Y., Furuta, T., Sugiyama, H., Hagihara, M., Uesugi, M. †
J. Am. Chem. Soc. 138, 9037-9040 (2016).
| =The G-quadruplexes form highly stable nucleic acid structures, which are implicated in various biological processes in both DNA and RNA. Although DNA G-quadruplexes have been studied in great detail, biological roles of RNA G-quadruplexes have received less attention. Here, a screening of a chemical library permitted identification of a small-molecule tool that binds selectively to RNA G-quadruplex structures. The polyaromatic molecule, RGB-1, stabilizes RNA G-quadruplex, but not DNA versions or other RNA structures. RGB-1 intensified the G-quadruplex-mediated inhibition of RNA translation in mammalian cells, decreased expression of the NRAS proto-oncogene in breast cancer cells, and permitted identification of a novel sequence that forms G-quadruplex in NRAS mRNA. RGB-1 may serve as a unique tool for understanding cellular roles of RNA G-quadruplex structures. | 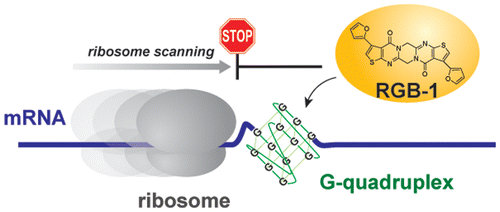 |
A potent and site-selective agonist of TRPA1. †
Takaya, J., Mio, K., Shiraishi, T., Kurokawa, T., Otsuka, S., Mori, Y., Uesugi, M. †
J. Am. Chem. Soc. 137, 15859−15864 (2015).
| =TRPA1 is a member of the transient receptor potential (TRP) cation channel family that is expressed primarily on sensory neurons. This chemosensor is activated through covalent modification of multiple cysteine residues with a wide range of reactive compounds including allyl isothiocyanate (AITC), a spicy component of wasabi. The present study reports on potent and selective agonists of TRPA1, discovered through screening 1657 electrophilic molecules. In an effort to validate the mode of action of hit molecules, we noted a new TRPA1-selective agonist, JT010 (molecule 1), which opens the TRPA1 channel by covalently and site-selectively binding to Cys621 (EC50 = 0.65 nM). The results suggest that a single modification of Cys621 is sufficient to open the TRPA1 channel. The TRPA1-selective probe described herein might be useful for further mechanistic studies of TRPA1 activation. | 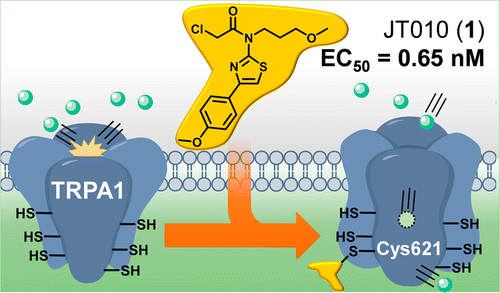 |
Intracellular generation of a diterpene-peptide conjugate that inhibits 14-3-3-mediated interactions. †
Parvatkar, P., Kato, N., Uesugi, M., Sato, S., Ohkanda, J. †
J. Am. Chem. Soc. 137, 15624-15627 (2015).
| =Synthetic agents that disrupt intracellular protein–protein interactions (PPIs) are highly desirable for elucidating signaling networks and developing new therapeutics. However, designing cell-penetrating large molecules equipped with the many functional groups necessary for binding to large interfaces remains challenging. Here, we describe a rational strategy for the intracellular oxime ligation-mediated generation of an amphipathic bivalent inhibitor composed of a peptide and diterpene natural product, fusicoccin, which binds 14-3-3 protein with submicromolar affinity. Our results demonstrate that co-treatment of cells with small module molecules, the aldehyde-containing fusicoccin 1 and the aminooxy-containing peptide 2, generates the corresponding conjugate 3 in cells, resulting in significant cytotoxicity. These results suggest that intracellular generation of large-sized molecules may serve as a new approach for modulating PPIs. | 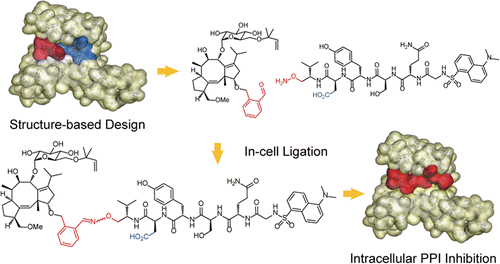 |
Live-cell imaging of endogenous mRNAs with a small molecule. †
Sato, S., Watanabe, M., Katsuda, Y., Murata, A., Wang, D. O., Uesugi M. †
Angew. Chem. Int. Ed. 54, 1855-1858 (2015).
Determination of subcellular localization and dynamics of mRNA is increasingly important to understanding gene expression. A new convenient and versatile method is reported that permits spatiotemporal imaging of specific non-engineered RNAs in living cells. The method uses transfection of a plasmid encoding a gene-specific RNA aptamer, combined with a cell-permeable synthetic small molecule, the fluorescence of which is restored only when the RNA aptamer hybridizes with its cognitive mRNA. The method was validated by live-cell imaging of the endogenous mRNA of β-actin. Application of the technology to mRNAs of a total of 84 human cytoskeletal genes allowed us to observe cellular dynamics of several endogenous mRNAs including arfaptin-2, cortactin, and cytoplasmic FMR1-interacting protein 2. The RNA-imaging technology and its further optimization might permit live-cell imaging of any RNA molecules.|#ref(research06.png,center,nolink,800x142)
Synthetic molecules that protect cells from anoikis and their use in cell transplantation. †
Frisco-Cabanos, H.L., Watanabe, M., Okumura, N., Kusamori, K., Takemoto, N., Takaya, J., Sato, S., Yamazoe, S., Takakura, Y., Kinoshita, S., Nishikawa, M., Koizumi, N., Uesugi, M. †
Angew. Chem. Int. Ed. 53, 11208-11213 (2014).
| =One of the major problems encountered in cell transplantation is the low level of survival of transplanted cells due to detachment-induced apoptosis, called anoikis. The present study reports on the chemical synthesis and biological evaluation of water-soluble molecules that protect suspended cells from anoikis. The synthetic molecules bind to and induce clusters of integrins and heparan-sulfate-bound syndecans, two classes of receptors that are important for extracellular matrix-mediated cell survival. Molecular biological analysis indicates that such molecules prolong the survival of suspended NIH3T3 cells, at least in part, by promoting clustering of syndecan-4 and integrin β1 on the cell surface, leading to the activation of small GTPase Rac-1 and Akt. In vivo experiments using animal disease models demonstrated the ability of the molecules to improve cell engraftment. The cluster-inducing molecules may provide a starting point for the design of new synthetic tools for cell-based therapy. | 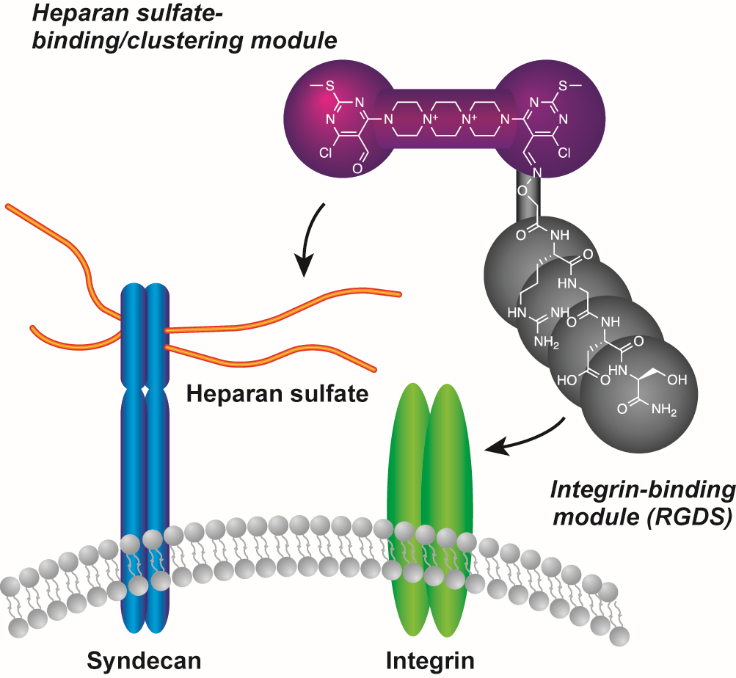 |
Selective elimination of human pluripotent stem cells by a marine natural product derivative. †
Kuo, T.F., Mao, D., Hirata, N., Khambu, B., Kimura, Y., Kawase, E., Shimogawa, H., Ojika, M., Nakatsuji, N., Ueda, K., Uesugi, M. †
J. Am. Chem. Soc. 136 (28), 9798-9801 (2014).
| =One of the current obstacles to stem cell therapy is the tumorigenic potential of residual undifferentiated stem cells. The present study reports rediscovery of a synthetic derivative of okadaic acid, a marine polyether toxin, as a reagent that selectively induces the death of human pluripotent stem cells. Cell-based screening of 333 cytotoxic compounds identified methyl 27-deoxy-27-oxookadaate (molecule 1) as a substrate of two ATP-binding cassette (ABC) transporters, ABCB1 (MDR1) and ABCG2 (BCRP), whose expression is repressed in human embryonic stem cells and induced pluripotent stem cells. The results demonstrate that selective elimination of human pluripotent stem cells can be achieved by designing cytotoxic small molecules with appropriate ABC-transporter selectivity. | 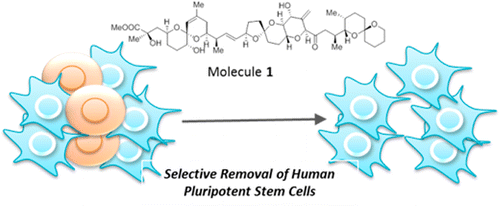 |
A chemical probe that labels human pluripotent stem cells. †
Hirata, N., Nakagawa, M., Fujibayashi, Y., Yamauchi, K., Murata, A., Minami, I., Tomioka, M., Kondo, T., Kuo, T.F., Endo, H., Inoue, H., Sato, S., Ando, S., Kawazoe, Y., Aiba, K., Nagata, K., Kawase, E., Chang, Y.T., Suemori, H., Eto, K., Nakauchi, H., Yamanaka, S., Nakatsuji, N., Ueda, K., Uesugi, M. †
Cell Rep 6., 1165–1174 (2014).
| =A small-molecule fluorescent probe specific for human pluripotent stem cells would serve as a useful tool for basic cell biology research and stem cell therapy. Screening of fluorescent chemical libraries with human induced pluripotent stem cells (iPSCs) and subsequent evaluation of hit molecules identified a fluorescent compound (Kyoto probe 1 [KP-1]) that selectively labels human pluripotent stem cells. Our analyses indicated that the selectivity results primarily from a distinct expression pattern of ABC transporters in human pluripotent stem cells and from the transporter selectivity of KP-1. Expression of ABCB1 (MDR1) and ABCG2 (BCRP), both of which cause the efflux of KP-1, is repressed in human pluripotent stem cells. Although KP-1, like other pluripotent markers, is not absolutely specific for pluripotent stem cells, the identified chemical probe may be used in conjunction with other reagents. | 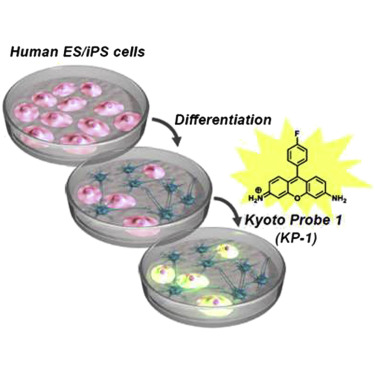 |
VMAT2 identified as a regulator of late-stage beta cell differentiation. †
Sakano, D., Shiraki, N., Kikawa, K., Yamazoe, T., Kataoka, M., Umeda, K., Araki, K., Mao, D., Matsumoto, S., Nakagata, N., Andersson, O., Stainier, D., Endo, F., Kume, K., Uesugi, M., Kume, S. †
Nat. Chem. Biol. 10, 141-148 (2014).
| =Cell replacement therapy for diabetes mellitus requires cost-effective generation of high-quality, insulin-producing, pancreatic β cells from pluripotent stem cells. Development of this technique has been hampered by a lack of knowledge of the molecular mechanisms underlying β-cell differentiation. The present study identified reserpine and tetrabenazine (TBZ), both vesicular monoamine transporter 2 (VMAT2) inhibitors, as promoters of late-stage differentiation of Pdx1-positive pancreatic progenitor cells into Neurog3 (referred to henceforth as Ngn3)-positive endocrine precursors. VMAT2-controlled monoamines, such as dopamine, histamine and serotonin, negatively regulated β-cell differentiation. Reserpine or TBZ acted additively with dibutyryl adenosine 3',5'-cyclic AMP, a cell-permeable cAMP analog, to potentiate differentiation of embryonic stem (ES) cells into β cells that exhibited glucose-stimulated insulin secretion. When ES cell–derived β cells were transplanted into AKITA diabetic mice, the cells reversed hyperglycemia. Our protocol provides a basis for the understanding of β-cell differentiation and its application to a cost-effective production of functional β cells for cell therapy. | 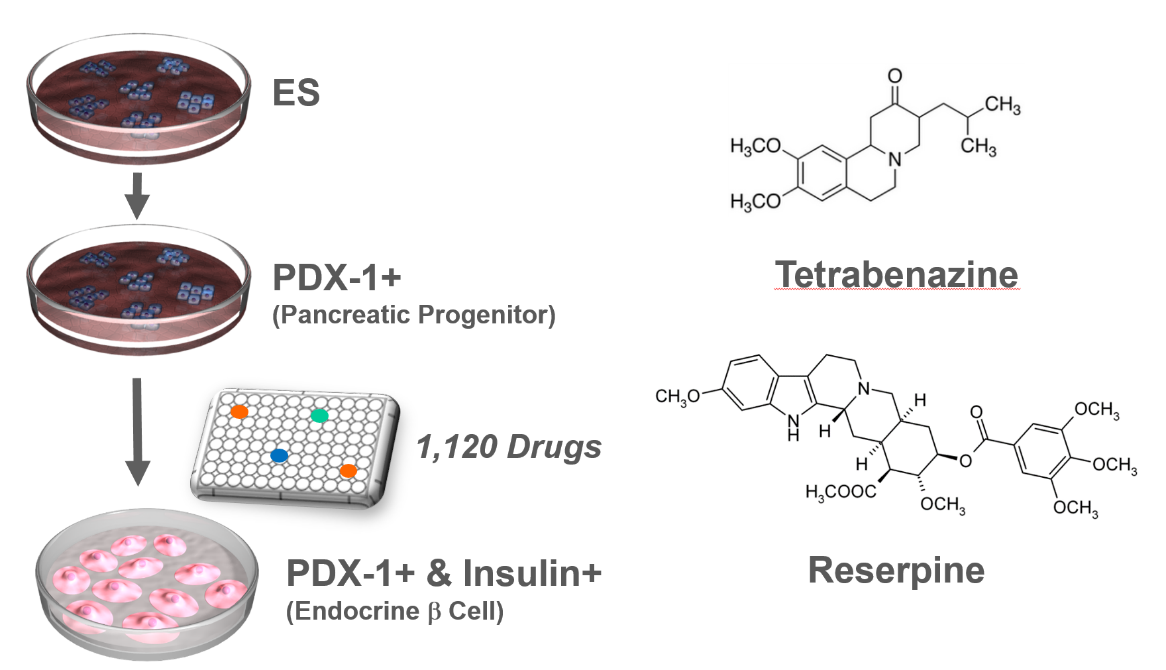 |
Inhibition of microtubule assembly by a complex of actin and antitumor macrolide aplyronine A. †
Kita, M., Hirayama, Y., Yoneda, K., Yamagishi, K., Chinen, T., Usui, T., Sumiya, E., Uesugi, M., Kigoshi, H. †
J. Am. Chem. Soc. 135 (48), 18089-18095 (2013).
| =Aplyronine A (ApA) is a marine natural product that shows potent antitumor activity. While both ApA and ApC, a derivative of ApA that lacks a trimethylserine ester moiety, inhibit actin polymerization in vitro to the same extent, only ApA shows potent cytotoxicity. Therefore, the molecular targets and mechanisms of action of ApA in cells have remained unclear. We report that ApA inhibits tubulin polymerization in a hitherto unprecedented way. ApA forms a 1:1:1 heterotrimeric complex with actin and tubulin, in association with actin synergistically binding to tubulin, and inhibits tubulin polymerization. Tubulin-targeting agents have been widely used in cancer chemotherapy, but there are no previous descriptions of microtubule inhibitors that also bind to actin and affect microtubule assembly. ApA inhibits spindle formation and mitosis in HeLa S3 cells at 100 pM, a much lower concentration than is needed for the disassembly of the actin cytoskeleton. The results of the present study indicate that ApA represents a rare type of natural product, which binds to two different cytoplasmic proteins to exert highly potent biological activities. | 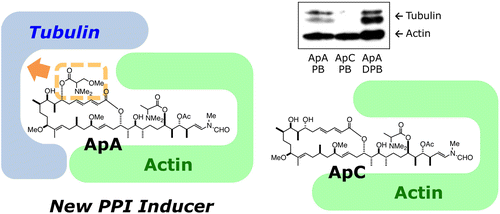 |
Small molecule-induced clustering of heparan sulfate promotes cell adhesion. †
Takemoto, N., Suehara, T., Frisco, H., Sato, S., Sezaki, T., Kusamori, K., Kawazoe, Y., Park, S., Yamazoe, S., Mizuhata, Y., Inoue, R., Miller, G., Hansen, S., Jayson, G., Gardiner, J., Kanaya, T., Tokitoh, N., Ueda, K., Takakura, Y., Kioka, N., Nishikawa, M., Uesugi, M. †
J. Am. Chem. Soc. 135 (30), 11032-11039 (2013).
| =Adhesamine is an organic small molecule that promotes adhesion and growth of cultured human cells by binding selectively to heparan sulfate on the cell surface. The present study combined chemical, physicochemical, and cell biological experiments, using adhesamine and its analogues, to examine the mechanism by which this dumbbell-shaped, non-peptidic molecule induces physiologically relevant cell adhesion. The results suggest that multiple adhesamine molecules cooperatively bind to heparan sulfate and induce its assembly, promoting clustering of heparan sulfate-bound syndecan-4 on the cell surface. A pilot study showed that adhesamine improved the viability and attachment of transplanted cells in mice. Further studies of adhesamine and other small molecules could lead to the design of assembly-inducing molecules for use in cell biology and cell therapy. | 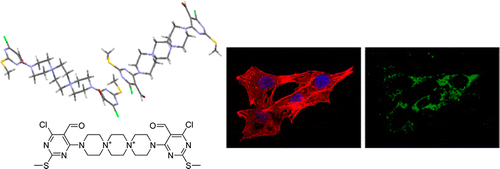 |
A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined cytokine- and xeno-free conditions. †
Minami, I., Yamada, K., Otsuji, T.G., Yamamoto, T., Shen, Y., Otsuka, S., Kadota, S., Morone, N., Barve, M., Asai, Y., Tenkova-Heuser, T., Heuser, J. E., Uesugi, M., Aiba, K., Nakatsuji, N. †
Cell Rep. (5), 1448-1460 (2012).
| =Human pluripotent stem cells (hPSCs), including embryonic stem cells and induced pluripotent stem cells, are potentially useful in regenerative therapies for heart disease. For medical applications, clinical-grade cardiac cells must be produced from hPSCs in a defined, cost-effective manner. Cell-based screening led to the discovery of KY02111, a small molecule that promotes differentiation of hPSCs to cardiomyocytes. Although the direct target of KY02111 remains unknown, results of the present study suggest that KY02111 promotes differentiation by inhibiting WNT signaling in hPSCs but in a manner that is distinct from that of previously studied WNT inhibitors. Combined use of KY02111 and WNT signaling modulators produced robust cardiac differentiation of hPSCs in a xeno-free, defined medium, devoid of serum and any kind of recombinant cytokines and hormones, such as BMP4, Activin A, or insulin. The methodology has potential as a means for the practical production of human cardiomyocytes for regeneration therapies. | 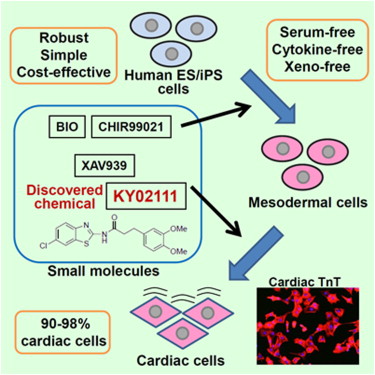 |
Synthesis and evaluation of diarylthiazole derivatives that inhibit activation of sterol regulatory element-binding proteins. †
Kamisuki, S., Shirakawa T., Kugimiya, A., Abu-Elheiga, L., Choo, H.-Y., Yamada, K., Shimogawa, H., Wakil, S. J., Uesugi, M. †
J. Med. Chem. 54(13), 4923-4927 (2011).
| =Fatostatin, a recently discovered small molecule that inhibits activation of sterol regulatory element-binding protein (SREBP), blocks biosynthesis and accumulation of fat in obese mice. We synthesized and evaluated a series of fatostatin derivatives. Our structure–activity relationships led to the identification of N-(4-(2-(2-propylpyridin-4-yl)thiazol-4-yl)phenyl)methanesulfonamide (24, FGH10019) as the most potent druglike molecule among the analogues tested. Compound 24 has high aqueous solubility and membrane permeability and may serve as a seed molecule for further development. | 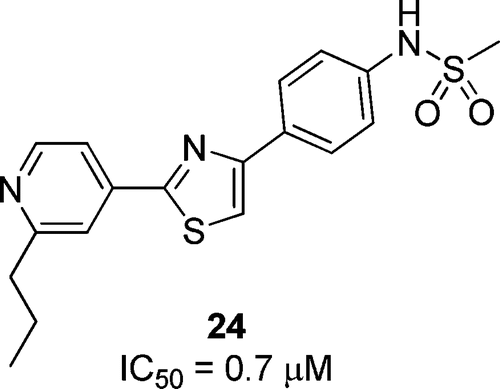 |
A mitochondrial surface-specific fluorescent probe activated by bioconversion. †
Kawazoe, Y., Shimogawa, H., Sato, A., Uesugi, M. †
Angew. Chem. Int. Ed. 50(24), 5478-5481 (2011).
Cell-based image screening of 12 000 small molecules with aromatic groups was carried out, and 31 were identified as having potential as fluorescent probes for living cells. One candidate is the first fluorescent probe that specifically stains mitochondrial surfaces. Spectroscopic analyses indicate that the molecule undergoes bioconversion to be fluorescent within cells.|#ref(research15.png,nolink,600x179)|
Small-molecule fluorescent probes for specific RNA targets. †
Murata, A., Sato, S., Kawazoe, Y., Uesugi, M. †
Chem. Comm. 47(16), 4712 - 4714 (2011).
| =A method was developed that uses small molecules as fluorescent probes to detect specific mRNAs. In this approach, the fluorescence of fluorophore-quencher conjugates is restored by the binding of an mRNA aptamer tag to the quencher segment of the molecules. The method allows real-time detection of mRNA transcripts in vitro. | 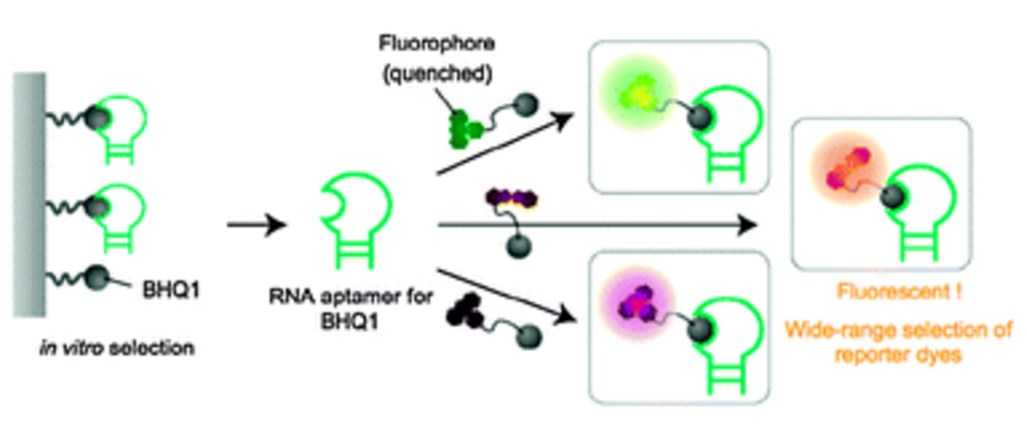 |
Cell-morphology profiling of a natural product library identifies bisebromoamide and miuraenamide A as actin-filament stabilizers. †
Sumiya, E., Shimogawa, H., Sasaki, H., Tsutsumi, M., Yoshita, K., Ojika, M., Suenaga, K., Uesugi, M. †
ACS Chem. Biol. 6(5), 425-431 (2011).
| =Natural products provide a rich source of biological tools, but elucidating their molecular targets remains challenging. Here we report a cell morphological profiling of a natural product library, which permitted the identification of bisebromoamide and miuraenamide A as actin filament stabilizers. Automated high-content image analysis showed that these two structurally distinct marine natural products induce morphological changes in HeLa cells similar to those induced by known actin-stabilizing compounds. Bisebromoamide and miuraenamide A stabilized actin filaments in vitro, and fluorescein-conjugated bisebromoamide localized specifically to actin filaments in cells. Cell morphological profiling was also used to identify actin-stabilizing or -destabilizing natural products from marine sponge extracts, leading to the isolation of pectenotoxin-2 and lyngbyabellin C. Overall, the results demonstrate that high-content imaging of nuclei and cell shapes offers a sensitive and convenient method for detecting and isolating molecules that target actin. | 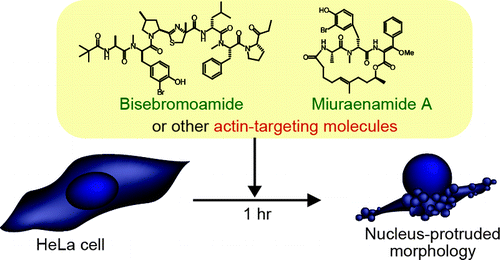 |
Deactivation of STAT6 through Serine 707 phosphorylation by JNK. †
Shirakawa, T. Kawazoe, Y., Tsujikawa, T., Jung, D., Sato, S., Uesugi, M. †
J. Biol. Chem. 286, 4003-4010 (2011).
| =Signal transducer and activator of transcription 6 (STAT6), which plays a critical role in immune responses, is activated by interleukin-4 (IL-4). Activity of STAT family members is regulated primarily by tyrosine phosphorylations and possibly also by serine phosphorylations. Here, we report a previously undescribed serine phosphorylation of STAT6, which is activated by cell stress or by the pro-inflammatory cytokine, interleukin-1β (IL-1β). Our analyses suggest that Ser-707 is phosphorylated by c-Jun N-terminal kinase (JNK). Phosphorylation decreases the DNA binding ability of IL-4-stimulated STAT6, thereby inhibiting the transcription of STAT6-responsive genes. Inactivation of STAT6 by JNK-dependent Ser-707 phosphorylation may be one mechanism of controlling the balance between IL-1β and IL-4 signals. | 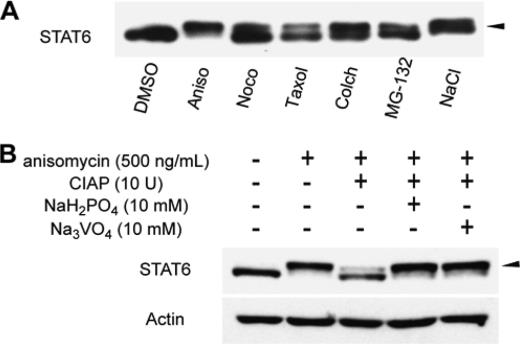 |
Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. †
Sato, S. Murata, A., Orihara, T., Shirakawa, T., Suenaga, K., Kigoshi, H., Uesugi, M. †
Chem. Biol. 18 (1), 131-139 (2011).
| =Aurilide is a potent cytotoxic marine natural product that induces apoptosis in cultured human cells at the picomolar to nanomolar range; however, its mechanism of action has been unknown. Results of the present study showed that aurilide selectively binds to prohibitin 1 (PHB1) in the mitochondria, activating the proteolytic processing of optic atrophy 1 (OPA1) and resulting in mitochondria-induced apoptosis. The mechanism of aurilide cytotoxicity suggests that PHB1 is an apoptosis-regulating protein amenable to modulation by small molecules. Aurilide may serve as a small-molecule tool for studies of mitochondria-induced apoptosis. | 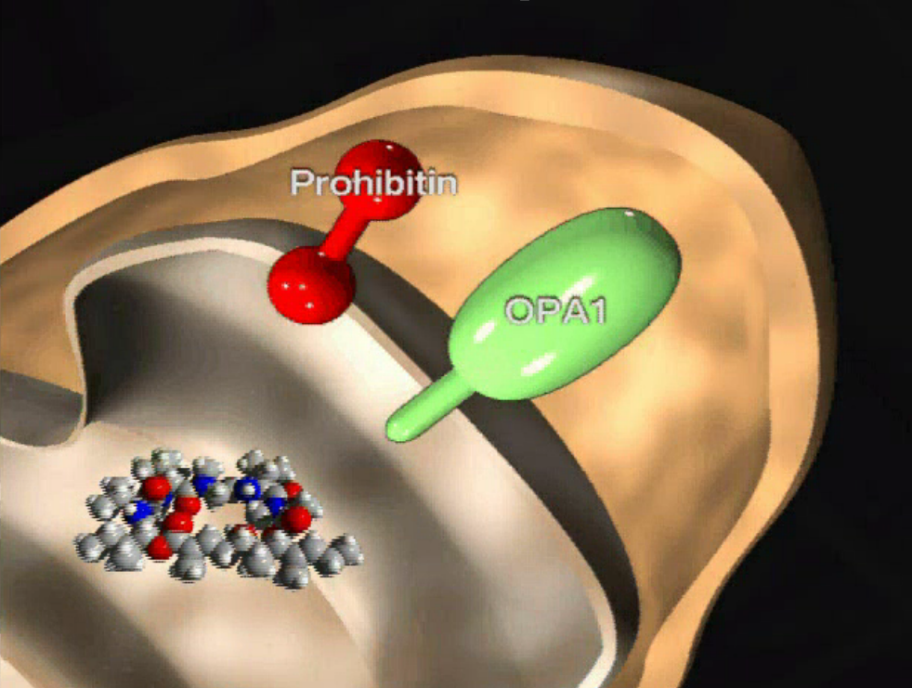 |
Biochemical target isolation for novices: Affinity-based strategies. (Review) †
Sato, S. Murata, A., Shirakawa, T., Uesugi, M. †
Chem. Biol. 17 (6), 616-623 (2010).
| =Although a number of genomic and biochemical technologies are now used to elucidate the mechanisms of action of bioactive small molecules, affinity-based isolation of molecular targets is a classic, but still powerful, approach. This review highlights recent cases where biochemical isolation of target proteins of bioactive small molecules highlighted general strategies for a successful isolation and identification of molecular targets. This review is intended to be both an update on the most recent findings for those already active in the field of forward chemical genetics and a guide for scientists entering this burgeoning field. | 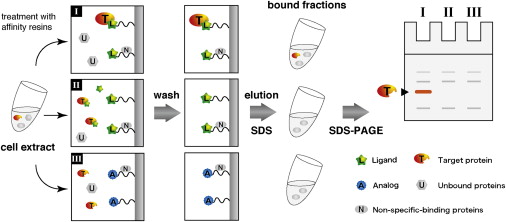 |
A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. †
Kamisuki, S., Mao, Q., Abu-Eliheiga, L., Gu, Z., Kugimiya, A., Kwon, Y., Shinohara, T., Kawazoe, Y., Sato, S. Asakura, K., Choo, H., Sakai, J., Wakil, SJ., Uesugi, M. †
Chem. Biol. 16 (8), 882-892 (2009).
| =Sterol regulatory element binding proteins (SREBPs) are transcription factors that activate transcription of the genes involved in cholesterol and fatty acid biosynthesis. In the present study, we show that a small synthetic molecule we previously discovered to block adipogenesis is an inhibitor of the SREBP activation. The diarylthiazole derivative, now called fatostatin, impairs the activation process of SREBPs, thereby decreasing the transcription of lipogenic genes in cells. Our analysis suggests that fatostatin inhibits the ER-Golgi translocation of SREBPs through binding to their escort protein, the SREBP cleavage-activating protein (SCAP), at a distinct site from the sterol-binding domain. Fatostatin blocked increases in body weight, blood glucose, and hepatic fat accumulation in obese ob/ob mice, even under uncontrolled food intake. Fatostatin may serve as a tool for gaining further insights into the regulation of SREBP. | 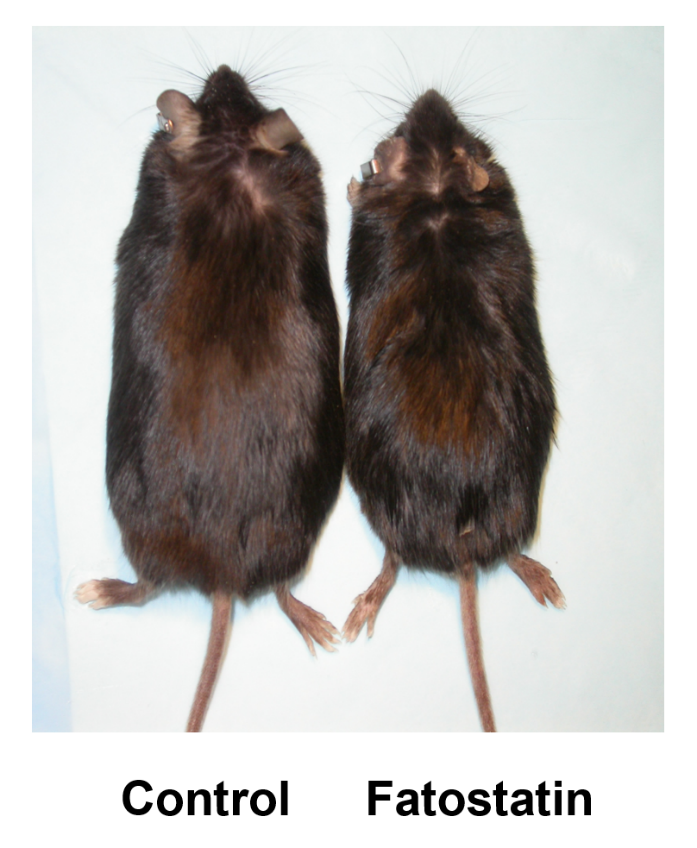 |
A dumbbell-shaped small molecule that promotes cell adhesion and growth. †
Yamazoe, S., Shimogawa, H., Sato, S., Esko, J. D., Uesugi, M. †
Chem. Biol. 16 (7), 773-782 (2009)
| =During an image-based phenotype screening of our chemical library, we noted a small molecule that boosts the adhesion and growth of human cells. Chemical and cell biological experiments suggest that the diaryldispirotripiperazine derivative (adhesamine) targets selective cell-surface glycosaminoglycans, especially heparan sulfate, for increasing cell adhesion and growth. The addition of adhesamine to the culture medium enables the adhesion of even floating lymphocytes to cell culture plates and the microinjection into them. Unlike poly-L-lysine, adhesamine induces apparently normal cell adhesion accompanied by organized actin structures and activation of focal adhesion kinase and ERK1/2 mitogen-activated protein kinases. Adhesamine may be useful as a cell-attaching reagent for cell engineering and basic cell biology. | 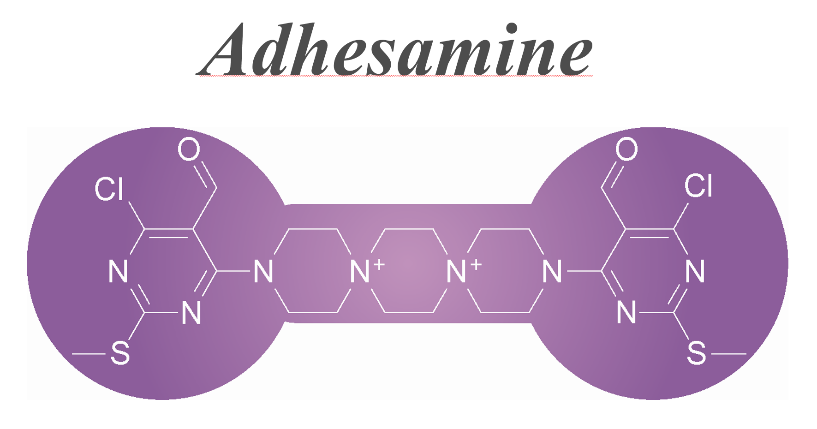 |
Wrenchnolol derivative optimized for gene activation in cells. †
Jung, D., Shimogawa, H., Kwon, Y., Mao, Q., Sato, S., Kamisuki, S., Kigoshi, H., Uesugi, M. †
J. Am. Chem. Soc. 131(13), 4774-4782 (2009)
| =Naturally occurring transcription factors usually have two independent domains, a DNA-binding domain and an activation domain. In designing a synthetic small molecule that mimics a transcription factor, each of the two domains needs to be replaced by small-molecule counterparts. Results of the present study show that derivatives of wrenchnolol, a synthetic molecule that interacts with Sur-2 coactivator, serve as activation modules and stimulate gene transcription in vitro and in cells when tethered to a DNA-binding molecule. Thirteen derivatives of wrenchnolol were chemically synthesized and tested for their ability to activate transcription in vitro and in cells. When tethered to the GAL4 DNA-binding domain, one derivative increased transcription of a GAL4-responsive reporter gene in cells 9-fold. This optimized derivative also induced up to 45% myogenesis of C2C12 cells when tethered to the DNA-binding domain of myogenic transcription factor MyoD. This optimized derivative may serve as a starting point for designing biological tools or components of fully synthetic transcription factors that permit selective up-regulation of genes. | 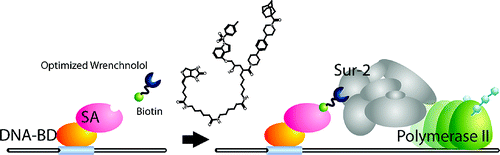 |
Polyproline-rod approach to isolating protein targets of bioactive small molecules: isolation of a new target of indomethacin. †
Sato, S., Kwon, Y., Kamisuki, S., Srivastava, N., Mao, Q., Kawazoe, Y., Uesugi, M. †
J. Am. Chem. Soc. 129(4), 873-880 (2007)
| =Identification of protein targets of bioactive small molecules has been a technical hurdle of chemical genetics. Here we report a polyproline-rod approach to isolating protein targets of small molecules from cell lysates. The results indicate that insertion of a long, rigid polyproline helix between a small-molecule bait and a biotin tag boosts the capacity of affinity purification and thereby permits isolation of low-abundance or low-affinity proteins. In the course of the proof-of-concept experiments, we isolated glyoxalase 1 (GLO1) as a new target of indomethacin, a widely used antiinflammatory drug. Molecular biological experiments suggest that inhibition of GLO1 enzyme activity is related to the clinically recognized beneficial side effects of the indomethacin family of nonsteroidal antiinflammatory drugs. | 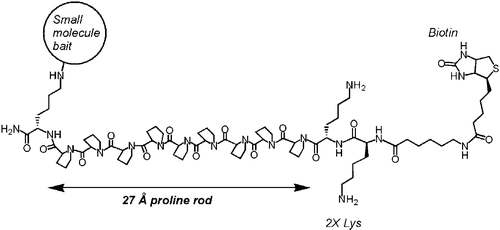 |
Chemical genetic identification of the IGF-linked pathway that is mediated by STAT6 and MFP2 †
Choi, Y., Shimogawa, H., Murakami, K., Ramdas, L., Zhang, W., Qin, J., Uesugi,M. †
Chem. Biol, 13, 241-249 (2006).
| =Insulin-like growth factor 2 (IGF2) is a potent mitogen whose deregulation plays a role in developing liver, breast, and prostate cancers. Here, we take a small-molecule approach to investigate molecular pathways that modulate IGF2 signaling, by using chromeceptin, a synthetic molecule that selectively impairs the viability and growth of IGF2-overexpressing hepatocellular carcinoma cells. Affinity purification revealed that chromeceptin binds to multifunctional protein 2 (MFP-2), a seemingly multifunctional enzyme implicated in peroxisomal β-oxidation. The small molecule-protein interaction stimulates the expression of IGF binding protein 1 (IGFBP-1) and suppressor of cytokine signaling-3 (SOCS-3), two cellular attenuators of the IGF signals, through activation of signal transducers and activators of transcription 6 (STAT6). The results underline the importance of STATs in IGF/insulin regulation, and they implicate a new pathway for STAT6 activation that is amenable to small-molecule intervention. | 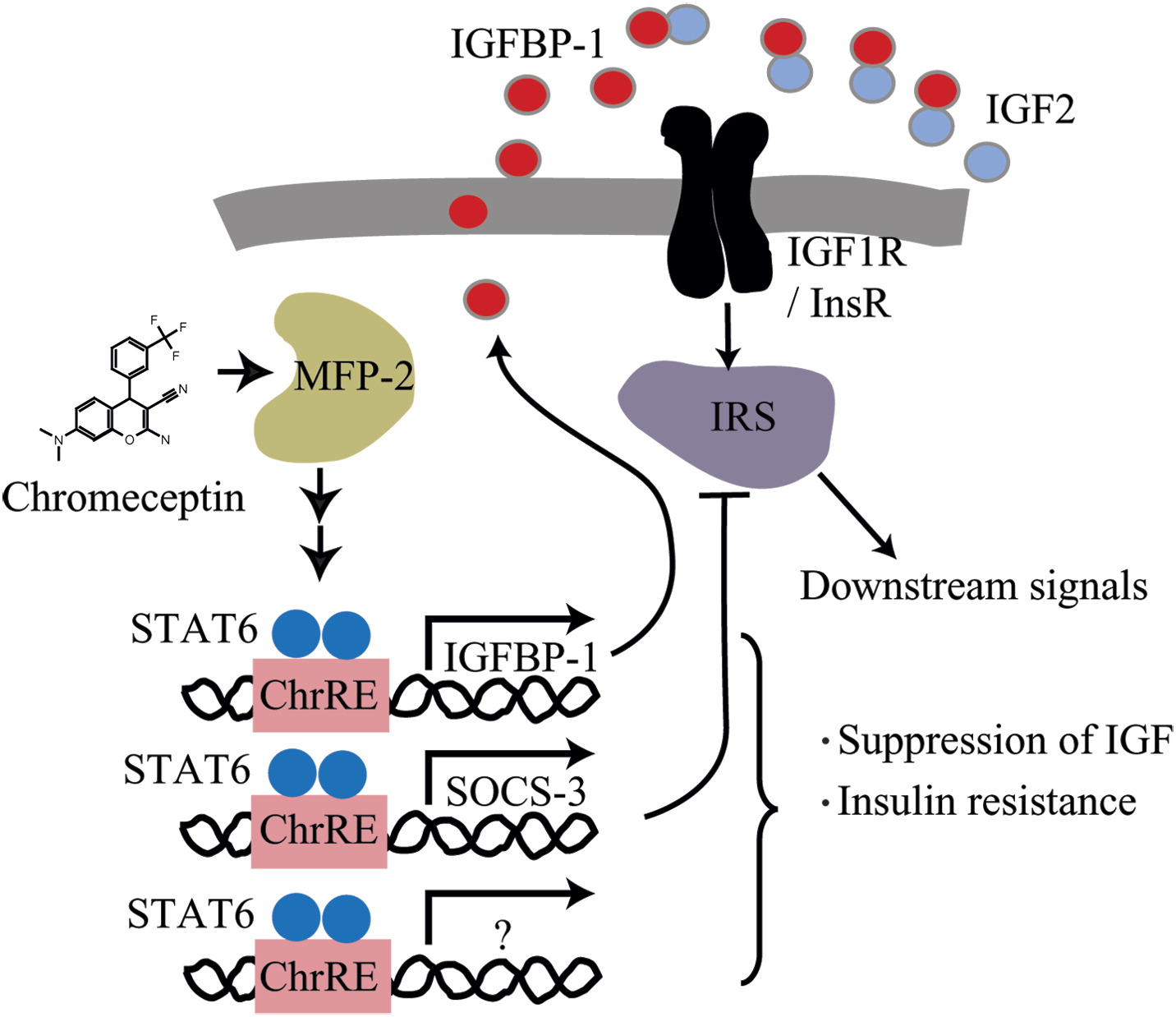 |
Chemical genetic identification of histamine receptor H1 as a stimulator of insulin-induced adipogenesis. †
Kawazoe, Y., Tanaka, H., Uesugi, M. †
Chem. Biol. 11, 907-913 (2004).
| =A large collection of bioactive compounds with diverse biological effects can be used as probes to elucidate new biological mechanisms that influence a particular cellular process. Here we analyze the effects of 880 well-known small-molecule bioactives or drugs on the insulin-induced adipogenesis of 3T3-L1 fibroblasts, a cell-culture model of fat cell differentiation. Our screen identified 86 compounds as modulators of the adipogenic differentiation of 3T3-L1 cells. Examination of their chemical and pharmacological information revealed that antihistamine drugs with distinct chemical scaffolds inhibit differentiation. Histamine H1 receptor is expressed in 3T3-L1 cells, and its knockdown by small interfering RNA impaired the insulin-induced adipogenic differentiation. Histamine receptors and histamine-like biogenic amines may play a role in inducing adipogenesis in response to insulin. | 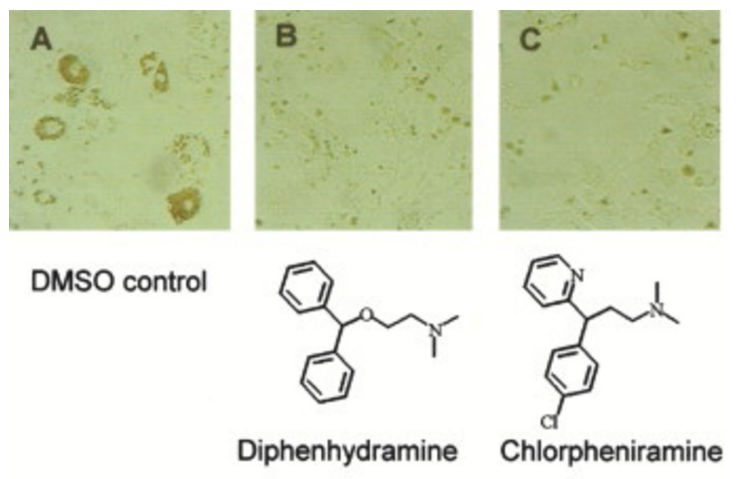 |
Small molecule transcription factor mimic. †
Kwon,Y., Arndt, H., Mao, Q., Choi, Y., Kawazoe, Y., Dervan, P. B., Uesugi, M. †
J. Am. Chem. Soc. 126, 15940-15941 (2004).
| =Regulation of gene expression by transcription factors touches many aspects of eukaryotic biology, and its systematic, external control by organic molecules represents a challenge in chemistry. Here we report the design of a completely organic, nonpeptidic compound that mimics a transcription factor. The design takes advantage of the specific DNA-binding affinity of a hairpin polyamide molecule and the ability of wrenchnolol to bind to the Sur-2 subunit of human mediator complex. The hybrid compound of these two molecules activates transcription of a reporter gene in vitro in a promoter-dependent manner through simultaneous contacts with DNA and Sur-2. Our results indicate that it is possible to generate a transcription factor out of nonpeptidic components. | 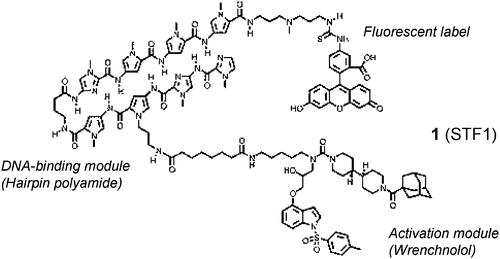 |
A wrench-shaped synthetic molecule that modulates a transcription factor-coactivator interaction. †
Shimogawa, H., Kwon, Y., Mao, Q., Kawazoe, Y., Choi, Y., Asada, S., Kigoshi, H., & Uesugi, M. †
J. Am. Chem. Soc. 126, 3461-3471 (2004).
| =Development of synthetic molecules that provide external control over the transcription of a given gene represents a challenge in medicinal and bioorganic chemistry. Here we report design and analysis of wrenchnolol, a wrench-shaped synthetic molecule that impairs the transcription of the Her2 oncogene by disrupting association of transcription factor ESX with its coactivator Sur-2. The “jaw” part of the compound mimics the α-helical interface of the activation domain of ESX, and the “handle” region accepts chemical modifications for a range of analysis. A water-soluble handle permitted NMR study in aqueous solution; a biotinylated handle verified the selectivity of the interaction, and a fluorescent handle confirmed the cell permeability of the compound. The case study of wrenchnolol foreshadows the promise and the challenge of targeting protein−protein interactions in the nucleus and may lead to the development of unique synthetic modulators of gene transcription. | 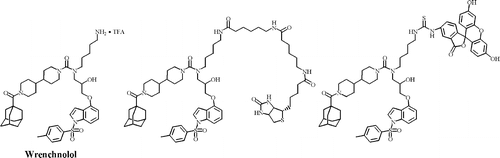 |
A gene-expression inhibitor that targets an alpha-helix-mediated protein interaction. †
Asada, S., Choi, Y., & Uesugi, M. †
J. Am. Chem. Soc. 125, 4992-4993 (2003).
| =Protein−protein interactions are harder to target by small organic molecules than by enzymes or nuclear hormone receptors. Here we report the discovery of an organic compound that inhibits the expression of the Her2 oncogene by disrupting an α-helix-mediated protein interaction. The druglike molecule we named adamanolol competitively inhibited the interaction between the two cancer-linked nuclear proteins, ESX (an epithelial-specific transcription factor) and Sur-2/DRIP130 (a Ras-linked subunit of the human mediator complex), which is important for the overexpression of Her2 gene in malignant breast cancer cells. Adamanolol impaired Her2 expression and caused cell death selectively in Her2-positive breast cancer cells. NMR signals of adamanolol suggest that its rigid conformation plays a role in forming a helixlike surface for the interaction. | 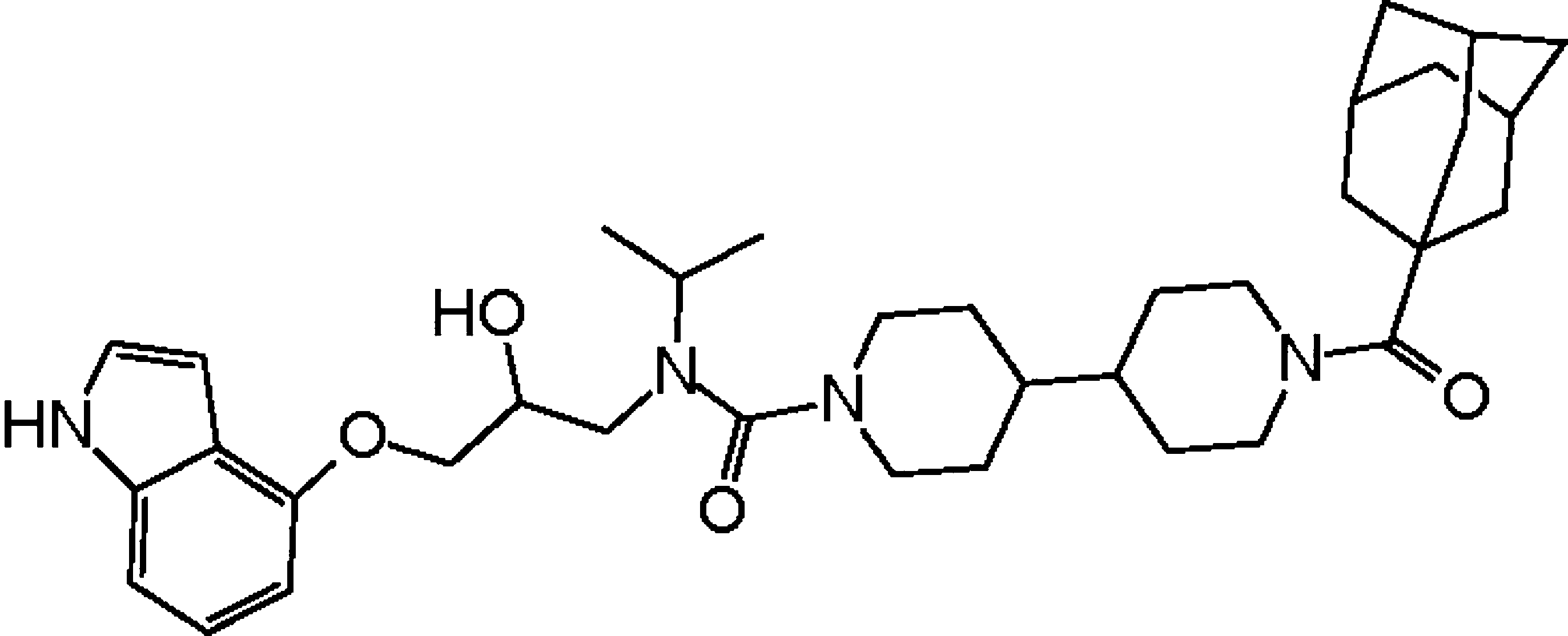 |
Identification of bioactive molecules by adipogenesis profiling of organic compounds. †
Choi, Y., Kawazoe, Y., Murakami, K., Misawa, H., & Uesugi, M †
J. Biol. Chem. 278, 7320-7324 (2003).
| =An important step in the postgenomic drug discovery is the construction of high quality chemical libraries that generate bioactive molecules at high rates. Here we report a cell-based approach to composing a focused library of biologically active compounds. A collection of bioactive non-cytotoxic chemicals was identified from a divergent library through the effects on the insulin-induced adipogenesis of 3T3-L1 cells, one of the most drastic and sensitive morphological alterations in cultured mammalian cells. The resulting focused library amply contained unique compounds with a broad range of pharmacological effects, including glucose-uptake enhancement, cytokine inhibition, osteogenesis stimulation, and selective suppression of cancer cells. Adipogenesis profiling of organic compounds generates a focused chemical library for multiple biological effects that are seemingly unrelated to adipogenesis, just as genetic screens with the morphology of fly eyes identify oncogenes and neurodegenerative genes. | 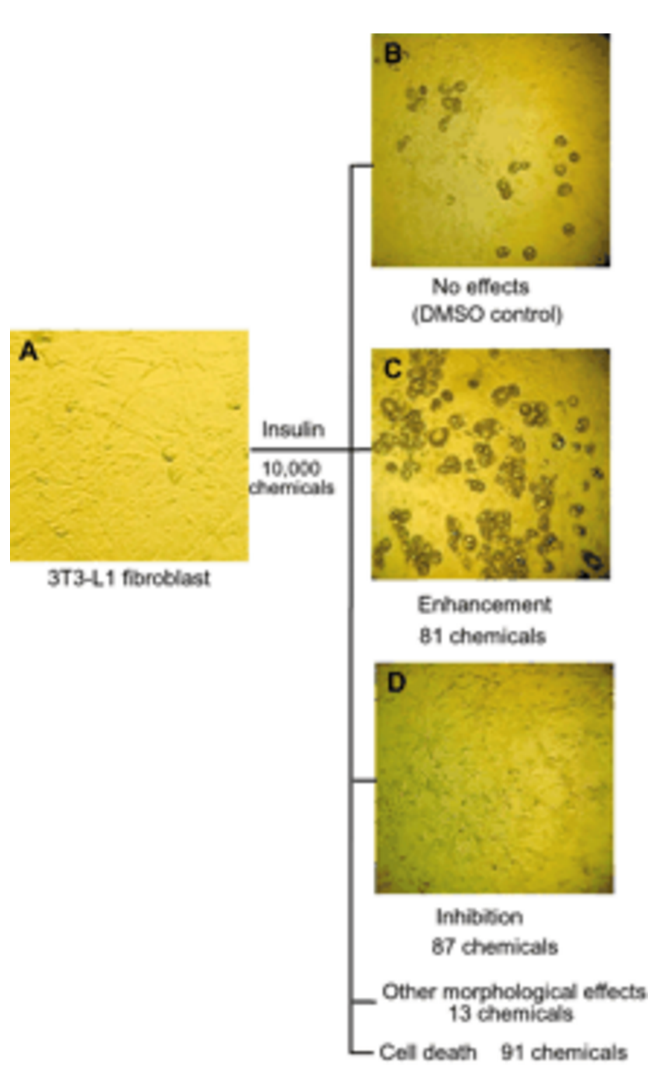 |
External control of Her2 expression and cancer cell growth by targeting a Ras-linked coactivator. †
Asada, S., Choi, Y., Yamada, M., Wang, S., Hung, M., Qin, J., & Uesugi, M. †
Proc. Natl. Acad. Sci. USA 99, 12747-12752 (2002).
| =Overproduction of the Her2 oncoprotein has been found in ≈30% of breast tumors, and patients who have Her2 excesses typically have more aggressive disease. Here we show that the expression of the Her2 gene can be decreased by inhibiting the interaction of the two cancer-linked proteins, DRIP130/CRSP130/Sur-2 (a Ras-linked subunit of human mediator complexes) and ESX (an epithelial-restricted transcription factor). Disruption of the interaction by a short cell-permeable peptide reduced the expression of the Her2 gene and specifically impaired the growth and viability of Her2-overexpressing breast cancer cells. The association of ESX with DRIP130 is mediated by a small hydrophobic face of an 8-aa helix in ESX, suggesting a therapeutic approach to incapacitating the Her2 gene by small organic molecules. | 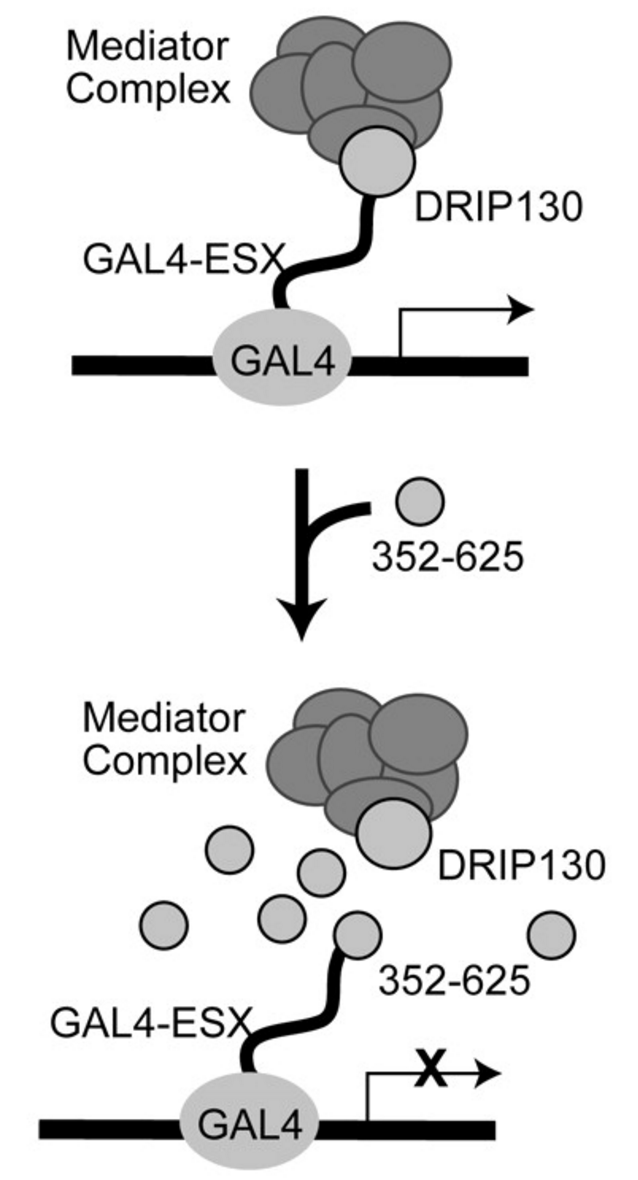 |
Divergent hTAF(II)31-binding motifs hidden in activation domains. †
Choi, Y., Asada, S., & Uesugi, M. †
J. Biol. Chem. 275, 15912-15916 (2000) .
| =Activation domains are functional modules that enable DNA-binding proteins to stimulate transcription. Characterization of these essential modules in transcription factors has been hampered by their low sequence homology. Here we delineate the peptide sequences that are required for transactivation and interaction with hTAFII31, a classical target of the acidic class of activation domains. Our analyses indicate that hTAFII31 recognizes a diverse set of sequences for transactivation. This information enabled the identification of hTAFII31-binding sequences that are critical for the activity of the activation domains of five human transcription factors: NFAT1, ALL1, NF-IL6, ESX, and HSF-1. The interaction surfaces are localized in short peptide segments of activation domains. The brevity and heterogeneity of the motifs may explain the low sequence homology among acidic activation domains. |  |
The alpha helical FXXphiphi motif in p53: TAF interaction and discrimination by MDM2. †
Uesugi, M. & Verdine, G. L. †
Proc. Natl. Acad. Sci. USA 96, 14801-14806 (1999) .
| =Transcriptional activation domains share little sequence homology and generally lack folded structures in the absence of their targets, aspects that have rendered activation domains difficult to characterize. Here, a combination of biochemical and nuclear magnetic resonance experiments demonstrates that the activation domain of the tumor suppressor p53 has an FXXPhiPhi motif (F, Phe; X, any amino acids; Phi, hydrophobic residues) that folds into an alpha-helix upon binding to one of its targets, hTAF(II)31 (a human TFIID TATA box-binding protein-associated factor). MDM2, the cellular attenuator of p53, discriminates the FXXPhiPhi motif of p53 from those of NF-kappaB p65 and VP16 and specifically inhibits p53 activity. Our studies support the notion that the FXXPhiPhi sequence is a general alpha-helical recognition motif for hTAF(II)31 and provide insights into the mechanistic basis for regulation of p53 function. | 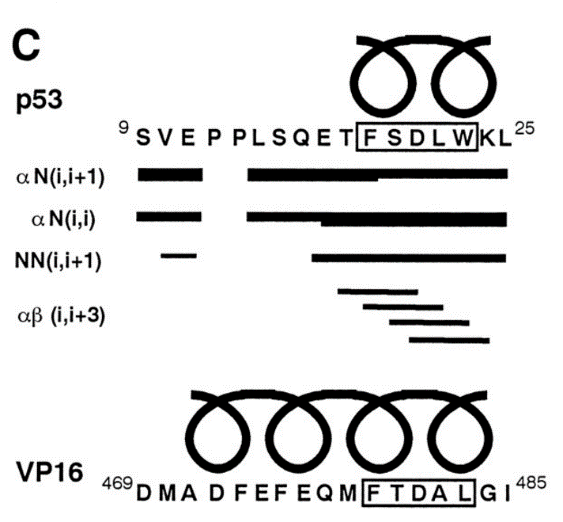 |
A non-natural transcriptional coactivator. †
Nyanguile, O., Uesugi, M., Austin, D. J., & Verdine, G. L. †
Proc. Natl. Acad. Sci. USA 94, 13402-13406 (1997) .
| =In eukaryotes, sequence-specific DNA-binding proteins activate gene expression by recruiting the transcriptional apparatus and chromatin remodeling proteins to the promoter through protein-protein contacts. In many instances, the connection between DNA-binding proteins and the transcriptional apparatus is established through the intermediacy of adapter proteins known as coactivators. Here we describe synthetic molecules with low molecular weight that act as transcriptional coactivators. We demonstrate that a completely nonnatural activation domain in one such molecule is capable of stimulating transcription in vitro and in vivo. The present strategy provides a means of gaining external control over gene activation through intervention using small molecules. | 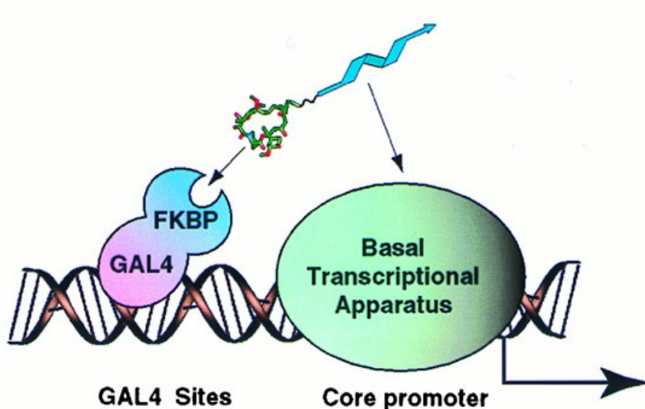 |
Induced alpha helix in the VP16 activation domain upon binding to a human TAF. †
Uesugi, M., Nyanguile, O., Lu, H., Levine, A. J., & Verdine, G. L. †
Science 277, 1310-1313 (1997) .
| =Activation domains are functional modules that enable sequence-specific DNA binding proteins to stimulate transcription. The structural basis for the function of activation domains is poorly understood. A combination of nuclear magnetic resonance (NMR) and biochemical experiments revealed that the minimal acidic activation domain of the herpes simplex virus VP16 protein undergoes an induced transition from random coil to α helix upon binding to its target protein, hTAFII31 (a human TFIID TATA box–binding protein-associated factor). Identification of the two hydrophobic residues that make nonpolar contacts suggests a general recognition motif of acidic activation domains for hTAFII31. | 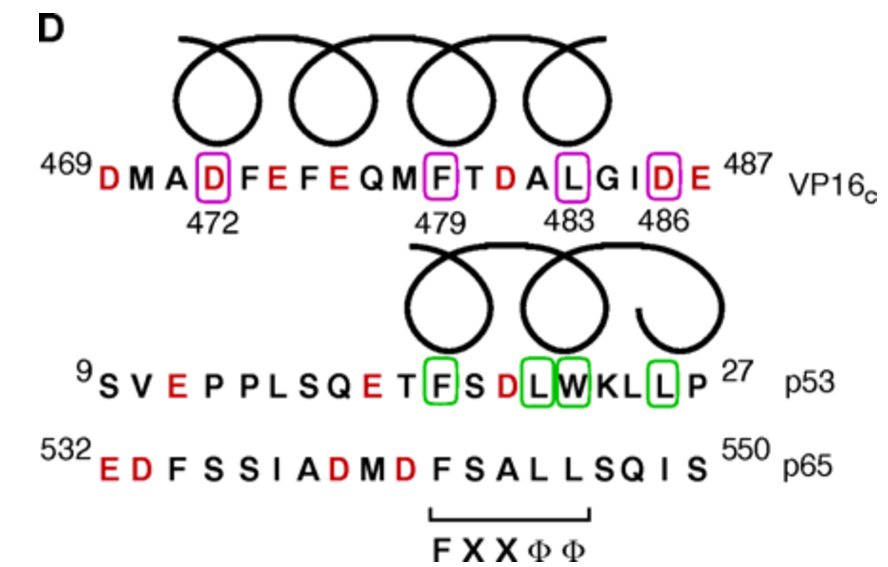 |
