
Research
We are working on the synthesis and development of various functional organic materials. In particular,
- Development of π-conjugated extended aromatic compounds using the precursor approach
- Development of integration processes for functional materials and expression of organic electronic properties
- Challenge of developing nanocarbon materials by substrate surface-assisted synthesis
- Development of novel materials by typical element chemistry
1. Development of π-extended aromatic compounds using the precursor approach
π-extended compounds are attractive as organic semiconductor materials and near-infrared dyes due to their electronic structure. However, in general, as the π-conjugation extends, their solubility in solvents decreases and their HOMO energy becomes shallower, making them more susceptible to oxidation and more difficult to synthesise. As a strategy to synthesise π-conjugated extended compounds with high purity, we have used the precursor method to synthesise various functional materials (Figure 1a) [1, 2]. For example, irradiating the α-diketone precursor of pentacene with visible light (ca. 460 nm) desorbs two molecules of CO, which can be quantitatively converted to acenes in solution, in thin films, in crystals and at low temperature (Figure 1b) [3].
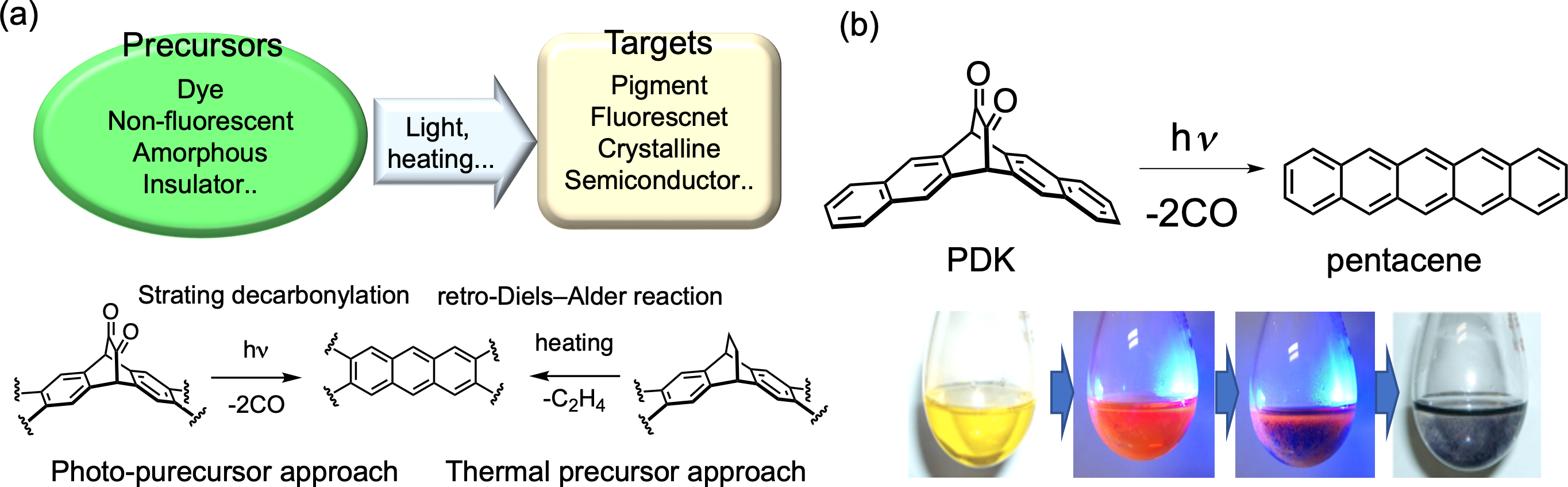
Figure 1. (a) Concept of “Precursor approach”, (b) Photo-conversion from the precursor, PDK, to pentacene in toluene.
The retro-Diels-Alder reaction by heating is also effective for conjugate extension of aromatic compounds. Using these precursor methods, various acenes and porphyrin-based functional organic materials have been developed (Figure 2) [4-6].
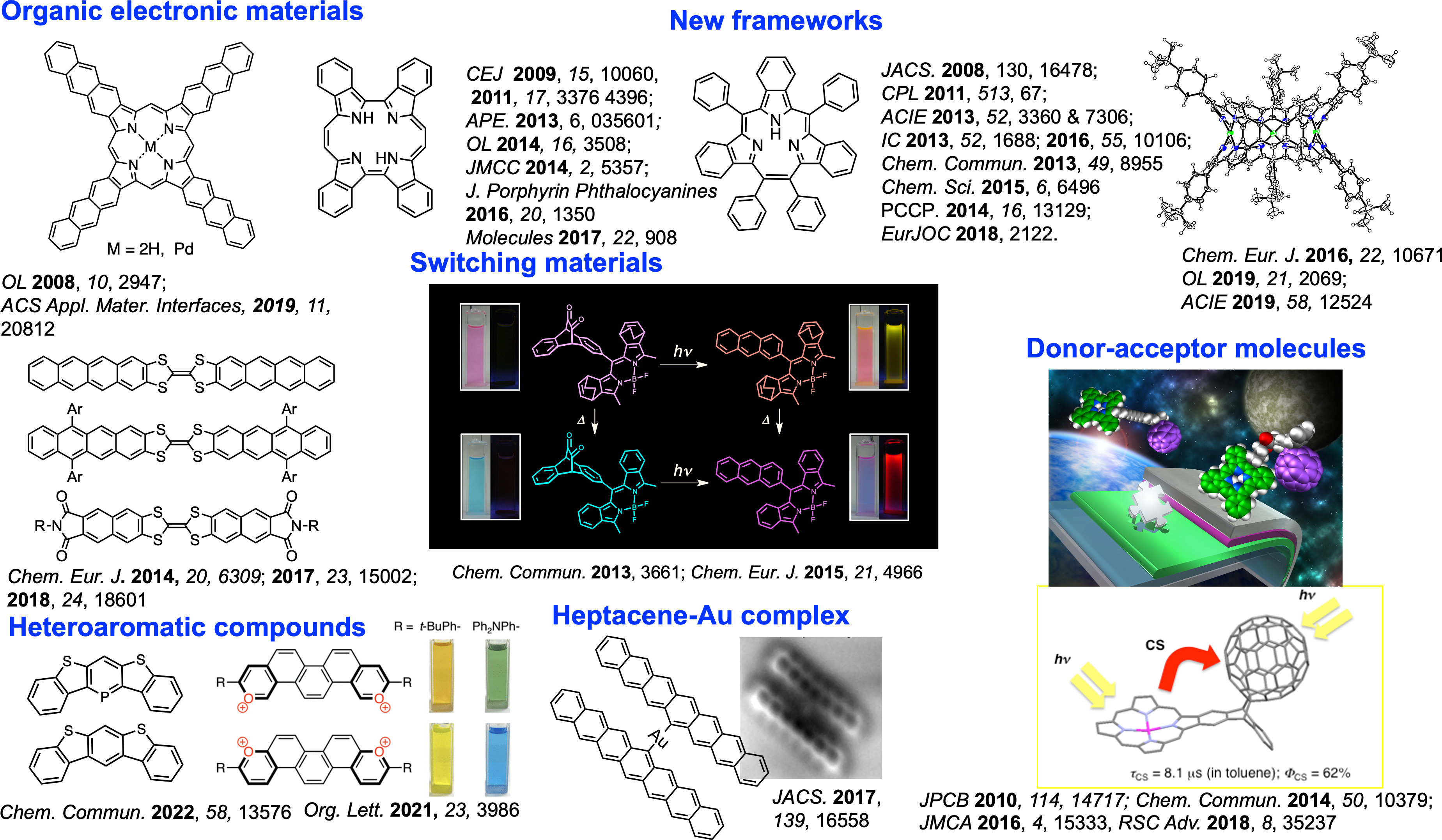
Figure 2. Examples of π-extended compounds prepared using “precursor approach”.
The reactions can be applied not only to the synthesis of insoluble or unstable π-conjugated extended compounds, but also to the development of deposition processes (see section 2). The reaction on the surface of a metal substrate under ultra-high vacuum has also led to the synthesis of higher acenes, which are difficult to synthesise by conventional methods, and to the elucidation of their electronic structure (see section 3).
References
- [1] M.Suzuki*. T. Aotake, Y. Yamaguchi, N. Noguchi, H. Nakano*, K. Nakayama*, and H. Yamada*, J. Photochem. Photobiol. C: Photochem. Reviews, 2014, 18, 50-70 (Review).
- [2] H. Yamada*, D. Kuzuhara, M. Suzuki, H. Hayashi, and N. Aratani Bull. Chem. Soc. Jpn. 2020, 93, 1234-1267 (Review).
- [3] H. Yamada* and H. Hayashi, Photochem. Photobiol. Sci. 2022, 21, 1311-1532 (Review).
- [4] K. Matsuo*, R. Okumura, H. Hayashi, N. Aratani, S. Jinnai, Y. Ie, A. Saeki, H. Yamada* Chem. Commun. 2022, 58, 13576-13579.
- [5] H. Yamada*, M. Yamashita, H. Hayashi, M. Suzuki, and N. Aratani, Chem. Eur. J., 2018, 24, 18601-18612 (Review).
- [6] D. Kuzuhara* and H. Yamada*, Synlett. 2021, 32, 1072-1084 (Review).
2. Development of integration processes for functional materials and expression of organic electronic properties
Organic field-effect transistors (OTFTs), organic thin-film solar cells (OPVs), and organic light-emitting diodes (OLEDs) using organic semiconductors are being actively studied as next-generation devices. Acenes such as pentacene are insoluble in common organic solvents and require vacuum deposition for thin film fabrication. Using the precursor method, solvent-soluble precursors can be deposited by spin-coating and then converted to a solvent-insoluble crystalline thin film by light irradiation or heating. The nanostructure inside the thin film and at the interface can be controlled, and the process can be repeated to realise multilayered structures by coating. We have successfully realised sandwich structures and controlled molecular orientation by coating, and consequently controlled the open circuit voltage (VOC) and short circuit current density (JSC) of OPVs (Figure 3) [1,2].
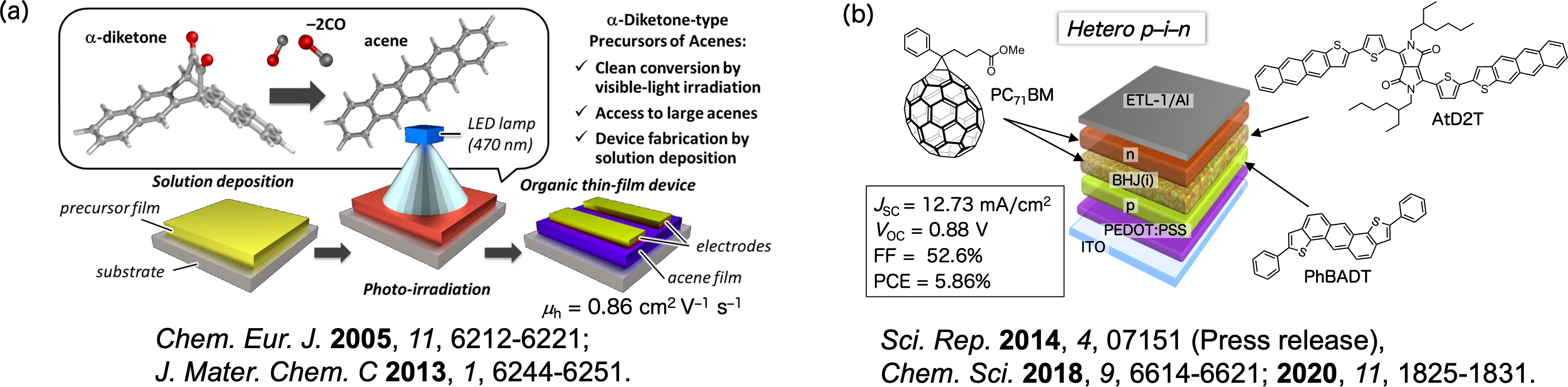
Figure 3. (a) FET evaluation using a coating process with pentacene photoprecursors; (b) organic thin-film solar cells with sandwich structures fabricated by the photoprecursor method.
Organic semiconductor materials usually exhibit hopping conduction, but it has recently been found that controlling the molecular framework, the packing structure, and the molecular orientation in the thin film enables band conduction and high charge mobility. For example, C8DMS-CuBP and C12DMS-CuBP films deposited by the simple dip-coat method show high FET properties of 4.3 cm2V-1s-1 and are expected to exhibit band conduction [3,4]. By improving the molecular backbone, substituents, central metal and deposition process of porphyrins, the molecular orientation can be controlled to achieve excellent organic electronic properties (Figure 4) [5].
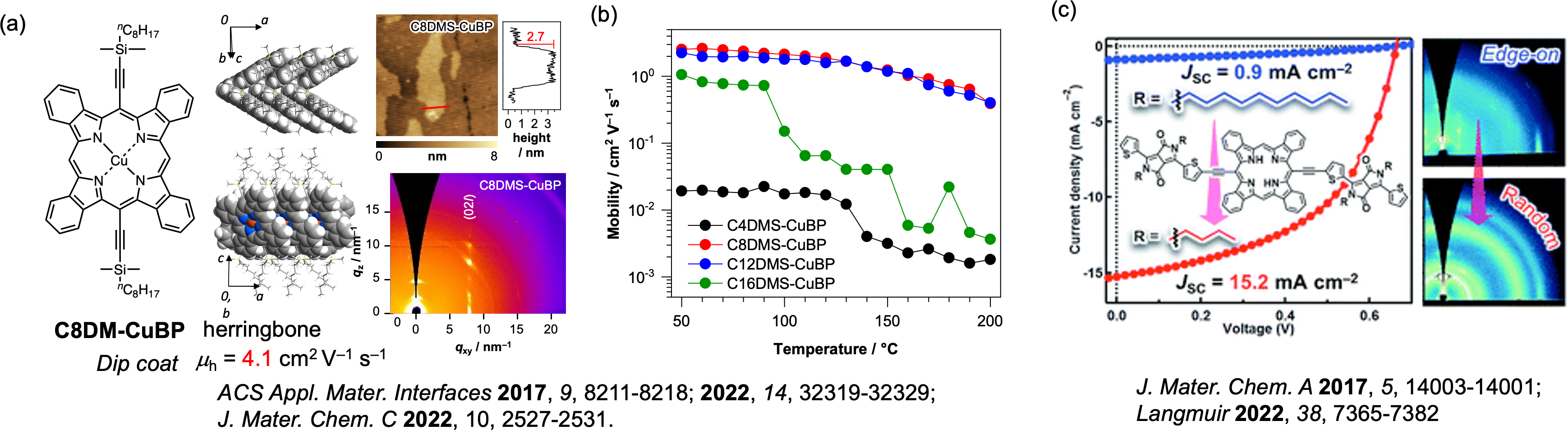
Figure 4. (a) Crystal structure of C8DMS-CuBP and FET charge carrier mobility deposited by the dip-coat method, AFM images and 2D-GIWAX image, (b) alkyl chain length dependence of thermal stability of charge carrier mobilities, and (c) alkyl chain length dependence of JSC of bulk hetero type OPVs prepared by the precursor method.
References
- [1] M. Suzuki,* Y. Yamaguchi, K. Uchinaga, K. Takahira, C. Quinton, S. Yamamoto, N. Nagami, M. Furukawa, K. Nakayama* and H. Yamada* Chem. Sci. 2018, 9, 6614-6621.
- [2] M. Suzuki,* K. Terai, C. Quinton, H. Hayashi, N. Aratani, H. Yamada* Chem. Sci. 2020, 11, 1825-1831.
- [3] K. Takahashi, B. Shan, X. Xu, S. Yang, T. Koganezawa, D. Kuzuhara, N. Aratani, M. Suzuki,* Q. Miao,* and H. Yamada* ACS Appl. Mater. Interfaces 2017, 9, 8211-8218.
- [4] E. Jeong, T. Ito, K. Takahashi, T. Koganezawa, H. Hayashi, N. Aratani, M. Suzuki*, and H. Yamada* ACS Appl. Mater. Interfaces 2022, 14, 32319-32329.
- [5] K. Takahashi, D. Kumagai, N. Yamada, D. Kuzuhara, Y. Yamaguchi, N. Aratani, T. Koganezawa, S. Koshika, N. Yoshimoto, S. Masuo, M. Suzuki,* K. Nakayama,* and H. Yamada* J. Mater. Chem. A 2017, 5, 14003-14011.
3. Challenge of developing nanocarbon materials by substrate surface-assisted synthesis
Higher acenes are the simplest polycyclic aromatic hydrocarbons with a linear structure of benzene rings. Higher acenes not only exhibit excellent charge transport properties comparable to those of amorphous silicon, but can also be key compounds for the synthesis of armchair graphene nanoribbons (GNRs) with a narrow band gap. Higher acenes, which can be regarded as the narrowest zigzag-type GNRs, are expected to be used as model systems to understand the edge states, chemical reactivities and electronic structure of zigzag-type GNRs. On the other hand, higher acenes are usually insoluble and unstable, and reports on their structural and physical properties are limited. Therefore, we aim to elucidate the correlation between the structure and physical properties of higher acenes and to develop reactions specific to substrate surface-assisted synthesis by combining the 'precursor method' and 'transformation reactions under anaerobic conditions' [1-3]. It has been experimentally demonstrated that nonacenes are open-shell biradicals in the ground state on Au(111) (Figure 5a). We are also developing precursors for the synthesis of cyclic cyclasenes (Figure 5b).
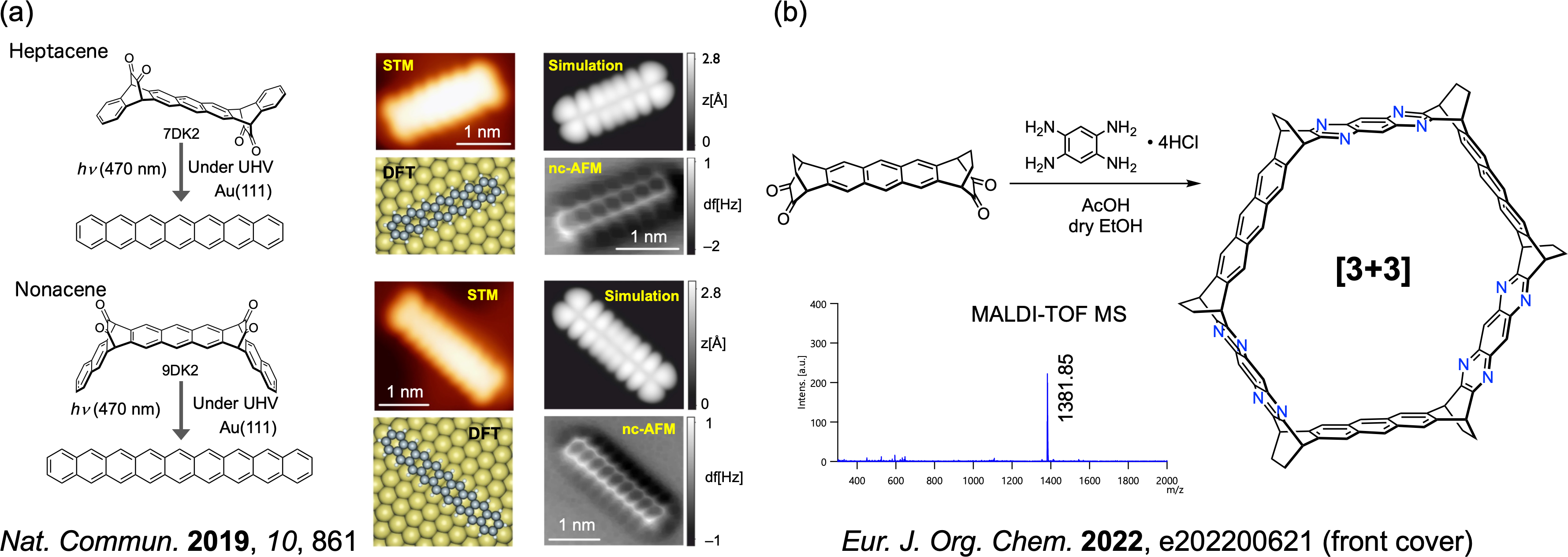
Figure 5. (a) Higher acene synthesis by substrate surface-assisted synthesis and electronic structure revealed by STM and nc-AFM; (b) Synthesis of azacyclacene precursors.
Graphene nanoribbons (GNRs) can be produced by a ring-fusion reaction of acene-polymers. Armchiar-type GNRs (AGNRs) are nanocarbon materials whose properties can be controlled by modifying their width and edge structures, and are expected to be key materials for next-generation organic devices. The width, length and edge structures of GNRs need to be controlled for practical use, and bottom-up synthesis methods are required. In collaboration with Fujitsu Laboratories (CREST project), we have worked on the synthesis of edge-modified AGNRs (Figure 6a) and wide GNRs, and succeeded in synthesising AGNRs with a band gap below 1.0 eV (Figure 6b) [4, 5], which is necessary for practical applications.

Figure 6. (a) Challenge to the fluorinated AGNR synthesis and the investigation of the fluorine desorption mechanism, (b) AGNR synthesis at gap energy 0.2 eV on Au(111) and the STM image.
References
- [1] J. I. Urgel, H. Hayashi, M. D. Giovannantonio, C. A. Pignedoli, S. Mishra, O. Deniz, M. Yamashita, T. Dienel, P. Ruffieux, H. Yamada*, and R. Fasel*, J. Am. Chem. Soc. 2017, 139, 11658-11661.
- [2] I. Urgel, S. Mishra, H. Hayashi, J. Wilhelm, C. A. Pignedoli, M. D. Giovannantonio, R. Widmer, M. Yamashita, N. Hieda, P. Ruffieux, H. Yamada*, and R. Fasel*, Nat. Commun. 2019, 10, 861.
- [3] K. Eimre*, José I. Urgel*, H. Hayashi, M. Di Giovannantonio, P. Ruffieux, S. Sato, S. Otomo, Y. S. Chan, N. Aratani, D. Passerone, O. Gröning, H. Yamada*, R. Fasel*, C. A. Pignedoli*, Nat. Commun. 2022, 13, 511.
- [4]H. Hayashi*, J. Yamaguchi, H. Jippo, R. Hayashi, N. Aratani, M. Ohfuchi, S. Sato*, and H. Yamada*, ACS Nano, 2017, 11, 6204-6210.
- [5] J. Yamaguchi*, H. Hayashi, H. Jippo, A. Shiotari, M. Ohtomo, M. Sakakura, N. Hieda, N. Aratani, M. Ohfuchi, Y. Sugimoto, H. Yamada*, and S. Sato, Commun. Mater., 2020, 1, 36 [press release, Top 15 of the Journal in 2020]
4. Development of novel materials by typical element chemistry
Compounds containing main group elements, especially higher-period elements (silicon, germanium, phosphorus, etc.), exhibit unique structures, reactivity, and physical properties not found in compounds of second-period elements such as carbon, nitrogen, and oxygen, which are treated in conventional organic chemistry. Therefore, they are attracting attention as a "treasure house of physical properties and functions" that can lead to the development of innovative materials.
We are working with typical elemental compounds using organic chemistry techniques to create new structures that bring out their excellent physical properties and reactivity. In particular, our research focuses on the synthesis and functional elucidation of π-electron compounds (multiply-bonded compounds and aromatic compounds) centered on high period group 14 elements such as silicon and germanium. These bonds are highly reactive and easily polymerize, but by utilizing "bulky substituents" (steric protection groups) developed in our laboratory, polymerization can be suppressed, and the compounds can be synthesized and isolated as compounds that can be handled at room temperature. Recently, we are constructing a method to stabilize such compounds without using steric oprotection groups, and are aiming for new developments.
In this way, we aim to contribute to the development of materials science through the creation of new material groups based on the extraction and establishment of "elemental properties" of typical elements and their application to functional materials, and through the development of comprehensive concepts for the systematic understanding of structures and reactions of typical element compounds.
page top