 | Research |

''
''
Create New World of Bioactive Synthetic Molecules
New ways to use, New Shapes, New sizes. †
As biological processes all stem from chemical events, it should be possible to understand or manipulate biological events by using chemistry. As chemical biologists our challenge is to discover or design unique organic molecules, 'super tools' that modulate fundamental processes in human cells. Using these 21st century super tools we can explore complex cellular events. Our job is creation: designing a new world of bioactive synthetic molecules, with unique and novel uses, forms and sizes. The future for small-molecule applications is open before us, in a range of fields, including future drug discovery and cell therapy. Our 21st century technology and skills will revolutionize the fight against the most difficult diseases and medical challenges of the age.
ケミカルバイオロジー~化学を起点とした生物学 †
ケミカルバイオロジーとは、化学を起点とした生物学です。生命の営みはせんじつめれば化学反応でできています。逆に化学を使って生命現象を理解したり、操ることができるはず。私たちの研究室では、生き物やヒト細胞にユニークな効果を及ぼす独自な有機化合物を見つける、もしくはデザインし、それらを道具として生命現象を探究・操作してきました。生物の仕組みは複雑ですが、有機化合物を起爆剤として用いることで、新たな切り口で生物を研究したり操作することができます。 私たちの大きな研究目標は、生理活性合成化合物の新しい世界を切り開くことです。生理活性化合物の新しい利用法、新しいカタチをした生理活性化合物、新しいサイズの生理活性化合物など、アイデアを与えることができればと思うのです。こういったアイデアは、未来の創薬の考え方や化合物の細胞治療への利用などに結びつくはずです。
''
''
Selected Publications †
Chemoproteomic Identification of Spermidine-Binding Proteins and Antitumor-Immunity Activators †
Vaibhav Pal Singh, Shuhei Hirose, Misao Takemoto, Asmaa M. A. S. Farrag, Shin-ichi Sato, Tasuku Honjo, Kenji Chamoto*, and Motonari Uesugi †
J. Am. Chem. Soc. 146(24), 16412–16418(2024)
https://doi.org/10.1021/jacs.3c14615

''
''
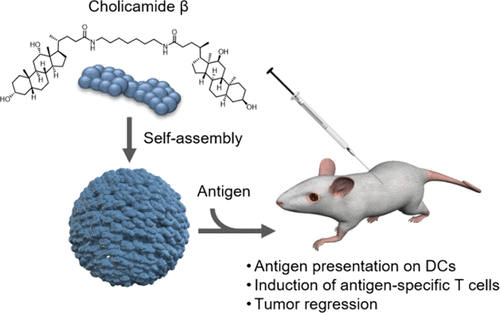
Identification of a Self-Assembling Small-Molecule Cancer Vaccine Adjuvant with an Improved Toxicity Profile †
Zhuo, S., Noda, N., Hioki, K., Jin, S., Hayashi, T., Hiraga, K., Momose, H., Li, W., Zhao, L., Mizukami, T., Ishii, K., Li, Y., Uesugi, M. †
J. Med. Chem. 66(18), 13266–13279 (2023)
https://doi.org/10.1021/acs.jmedchem.3c01252
''
''
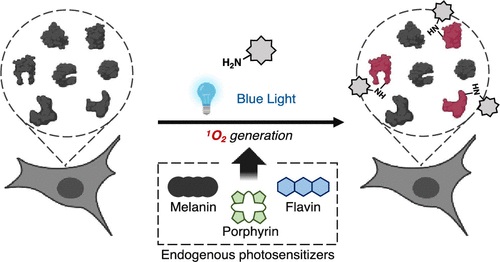
Chemoproteomic Identification of Blue-Light-Damaged Proteins †
Toh, K., Nishio, K., Nakagawa, R., Egoshi, S., Abo, M., Perron, A., Sato, S., Okumura, N., Koizumi, N., Dodo, K., Sodeoka, M., Uesugi, M. †
J. Am. Chem. Soc. 144(44), 20171–20176 (2022)
https://doi.org/10.1021/jacs.2c07180
''
''
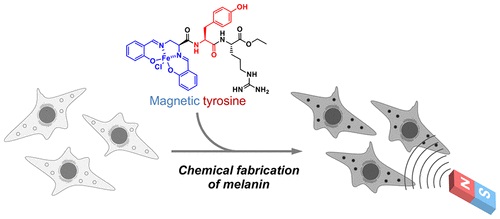
Magnetic Control of Cells by Chemical Fabrication of Melanin †
Nishio, K., Toh, K., Perron, A., Goto, M., Abo, M., Shimakawa, Y., Uesugi, M. †
J. Am. Chem. Soc. 144(37), 16720–16725 (2022)
https://doi.org/10.1021/jacs.2c06555
''
''
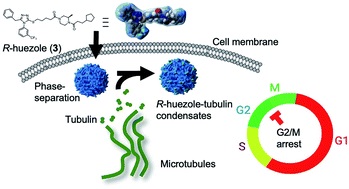
Discovery of a phase-separating small molecule that selectively sequesters tubulin in cells †
Ado, G., Noda, N., Vu, H., Perron, A., Mahapatra, A., Arista, K., Yoshimura, H., Packwood, D., Ishidate, F., Sato, S., Ozawa, T., Uesugi, M. †
Chemical Science 13, 5760-5766 (2022)
https://doi.org/10.1039/D1SC07151C
''
''
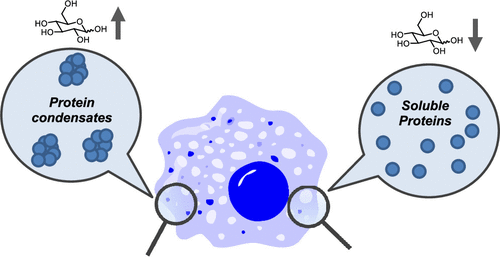
Glucose as a Protein-Condensing Cellular Solute †
Noda, N., Jung, Y., Ado, G., Mizuhata, Y., Higuchi, M., Ogawa, T., Ishidate, F., Sato, S., Kurata, H., Tokitoh, N., Uesugi, M. †
ACS Chem. Biol. 17(3), 567–575 (2022)
https://doi.org/10.1021/acschembio.1c00849
''
''
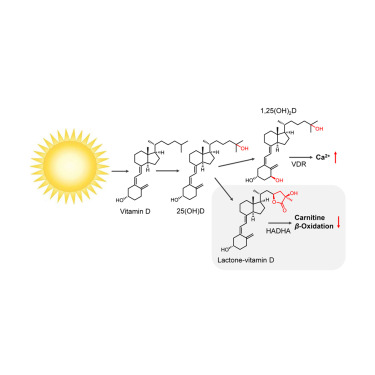
Controlled lipid β-oxidation and carnitine biosynthesis by a vitamin D metabolite. †
Mendoza, A., Takemoto, Y., Cruzado, K., Masoud, S., Nagata, A., Tantipanjaporn, A., Okuda, S., Kawagoe, F., Sakamoto, R., Odagi, M., Mototani, S., Togashi, M., Kawatani, M., Aono, H., Osada, H., Nakagawa, H., Higashi, T., Kittaka, A., Nagasawa, K., Uesugi, M. †
Cell Chemical Biology 29(4), 660-669 (2022)
https://doi.org/10.1016/j.chembiol.2021.08.008
''
''
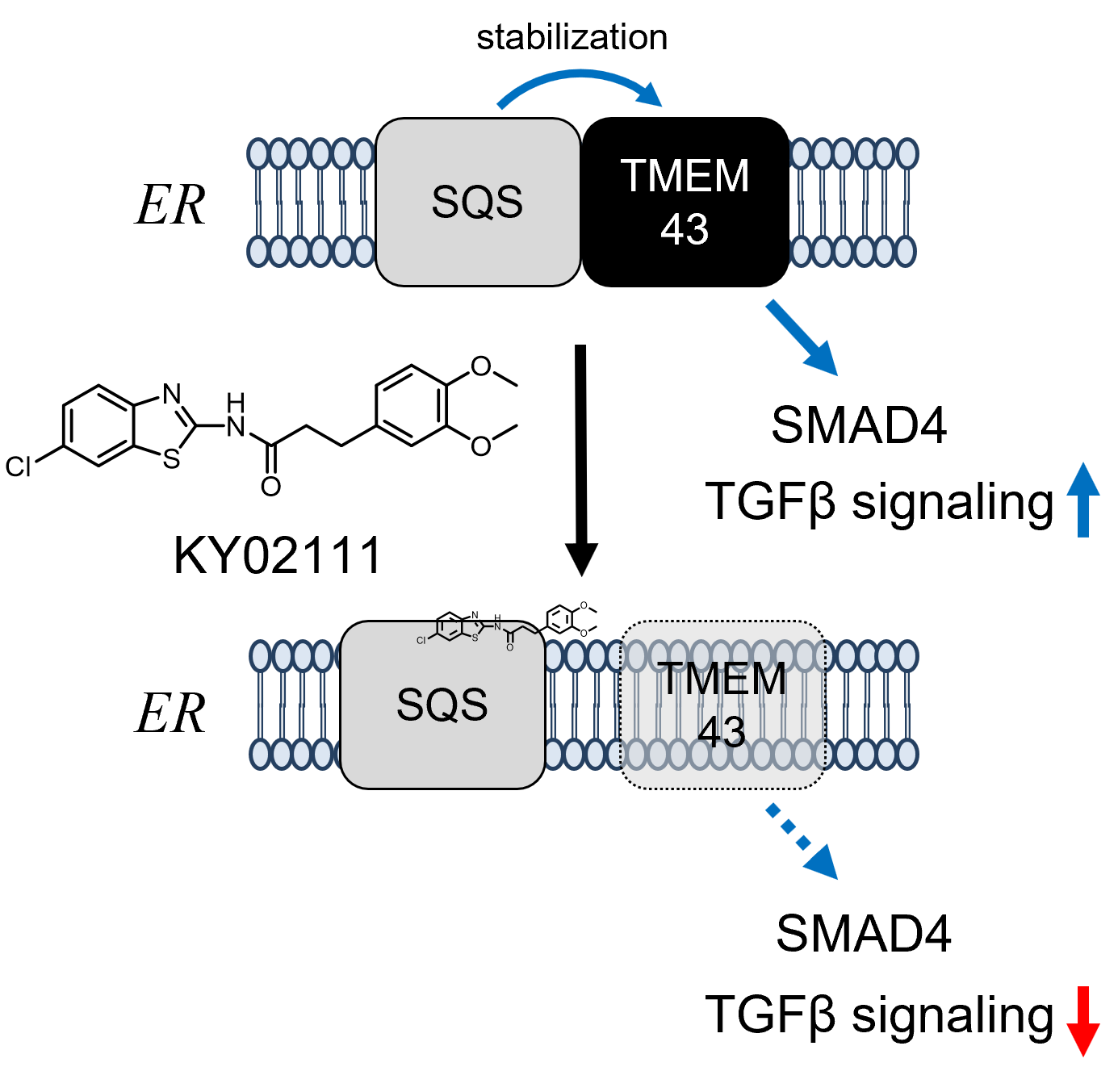
Chemical Genetics Reveals a Role of Squalene Synthase in TGFβ Signaling and Cardiomyogenesis †
Takemoto, Y., Kadota, S., Minami, I., Otsuka, S., Okuda, S., Abo, M., Punzalan, L., Shen, Y., Shiba, Y., Uesugi, M. †
Angew. Chem. Int. Ed. 60(40), 21824-21831 (2021)
https://doi.org/10.1002/anie.202100523
''
''
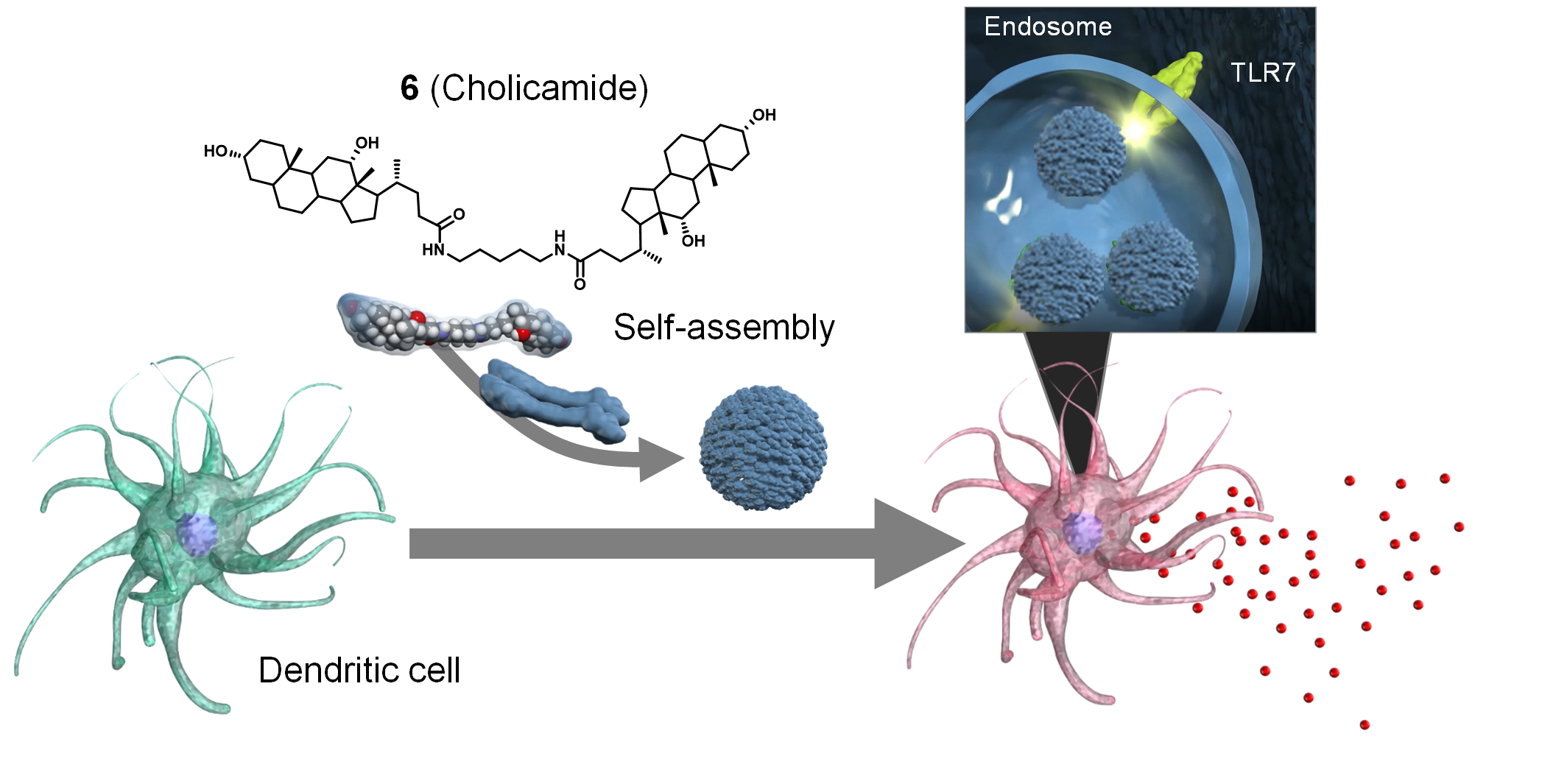
Discovery of Self‐Assembling Small Molecules as Vaccine Adjuvants. †
Jin, S., Vu, H., Hioki, K., Noda, N., Yoshida, H., Shimane, T., Ishizuka, S., Takashima, I., Mizuhata, Y., Pe, K., Ogawa, T., Nishimura, N., Packwood, D., Tokitoh, N., Kurata, H., Yamasaki, S., Ishii, K., Uesugi, M. †
Angew. Chem. Int. Ed. 60(2), 961-969 (2021)
https://doi.org/10.1002/anie.202011604
''
''
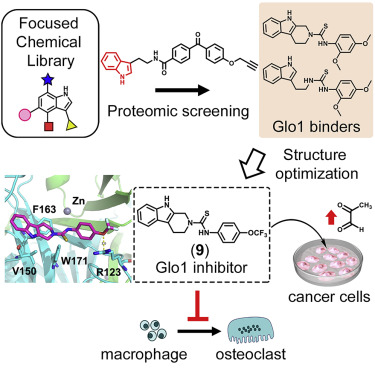
Chemoproteomic Profiling of a Pharmacophore-Focused Chemical Library. †
Punzalan, L., Jiang, L., Mao, D., Mahapatra, A., Sato, S., Takemoto, Y., Tsujimura, M., Kusamori, K., Nishikawa, M., Zhou, L., Uesugi, M. †
Cell Chemical Biology 27(6), 708–718 (2020)
https://doi.org/10.1016/j.chembiol.2020.04.007
''
''
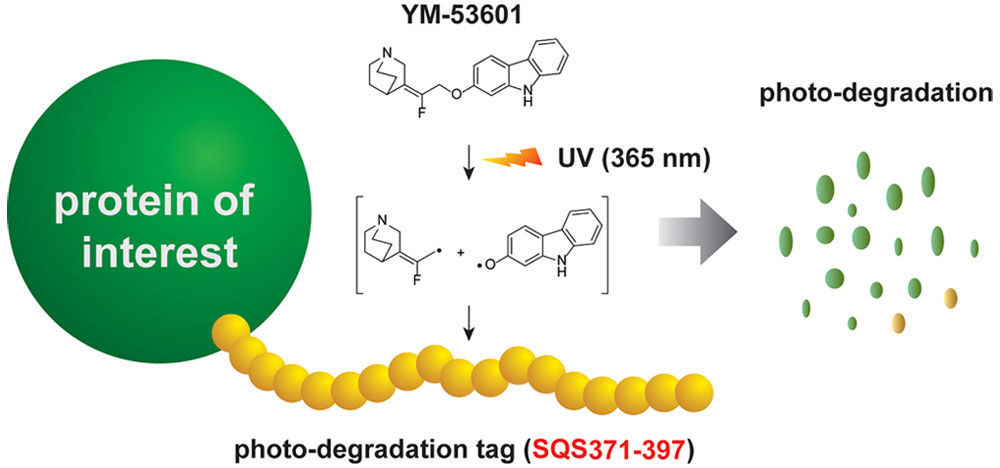
Discovery of a Small-Molecule-Dependent Photolytic Peptide. †
Takemoto, Y., Mao, Di., Punzalan, L., Götze, S., Sato, S., Uesugi, M. †
J. Am. Chem. Soc. 142(3), 1142-1146 (2020)
https://doi.org/10.1021/jacs.9b09178
''
''
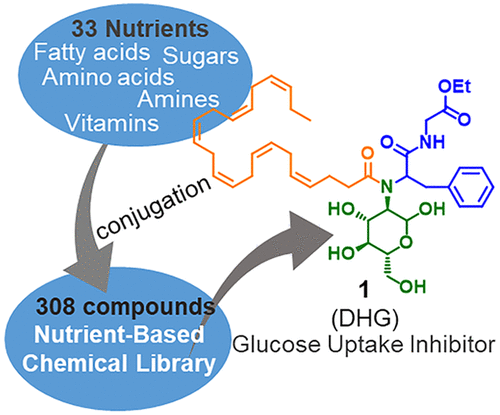
Nutrient-Based Chemical Library as a Source of Energy Metabolism Modulators. †
Furuta,T., Mizukami, Y., Asano, L., Kotake, K., Ziegler, S., Yoshida, H., Watanabe, M., Sato, S., Waldmann, H., Nishikawa, M., Uesugi, M. †
ACS Chem. Biol. 14(9), 1860-1865 (2019)
https://doi.org/10.1021/acschembio.9b00444
''
''
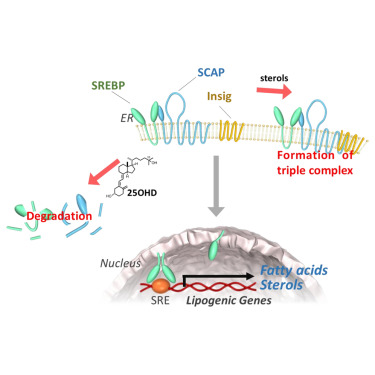
Vitamin D metabolite, 25-Hydroxyvitamin D, regulates lipid metabolism by inducing degradation of SREBP/SCAP. †
Asano, L., Watanabe, M., Ryoden, Y., Usuda, K., Yamaguchi, T., Khambu, B., Takashima, M., Sato, S., Sakai, J., Nagasawa, K., Uesugi, M. †
Cell Chem Biol. 24, 207-217 (2017)
https://doi.org/10.1016/j.chembiol.2016.12.017
''
''
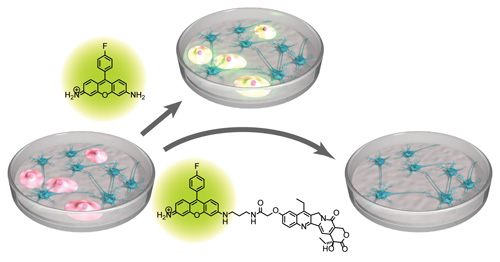
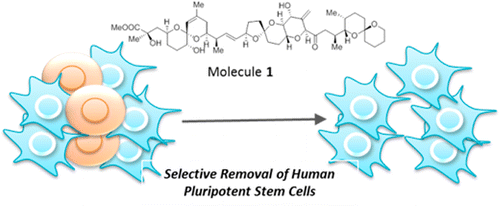
A synthetic hybrid molecule for the selective removal of human pluripotent stem cells from cell mixtures. †
Mao, D., Ando, S., Sato, S., Qin, Y., Hirata, N., Katsuda, Y., Kawase, E., Kuo, T.F., Minami, I., Shiba, Y., Ueda, K., Nakatsuji, N., Uesugi, M. †
Angew. Chem. Int. Ed. 56(7), 1765-1770 (2017)
https://doi.org/10.1002/anie.201610284
''
''
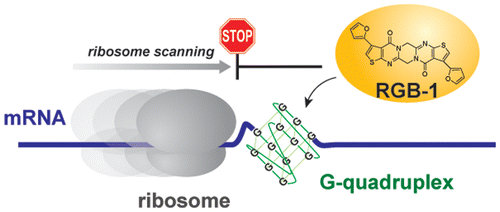
A small molecule that represses translation of G-quadruplex-containing mRNA. †
Katsuda, Y., Sato, S., Asano, L., Morimura, Y., Furuta, T., Sugiyama, H., Hagihara, M., Uesugi, M. †
J. Am. Chem. Soc. 138, 9037-9040 (2016)
https://doi.org/10.1021/jacs.6b04506
''
''
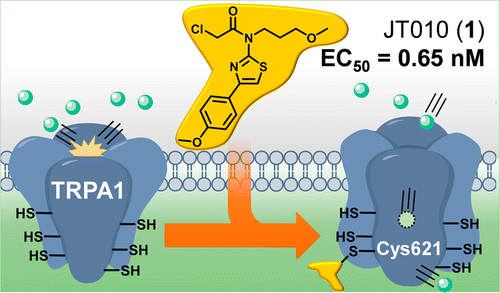
A potent and site-selective agonist of TRPA1. †
Takaya, J., Mio, K., Shiraishi, T., Kurokawa, T., Otsuka, S., Mori, Y., Uesugi, M. †
J. Am. Chem. Soc. 137, 15859−15864 (2015)
https://doi.org/10.1021/jacs.5b10162
''
''
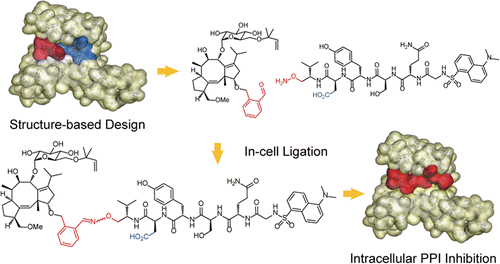
Intracellular generation of a diterpene-peptide conjugate that inhibits 14-3-3-mediated interactions. †
Parvatkar, P., Kato, N., Uesugi, M., Sato, S., Ohkanda, J. †
J. Am. Chem. Soc. 137, 15624-15627 (2015)
https://doi.org/10.1021/jacs.5b09817
''
''
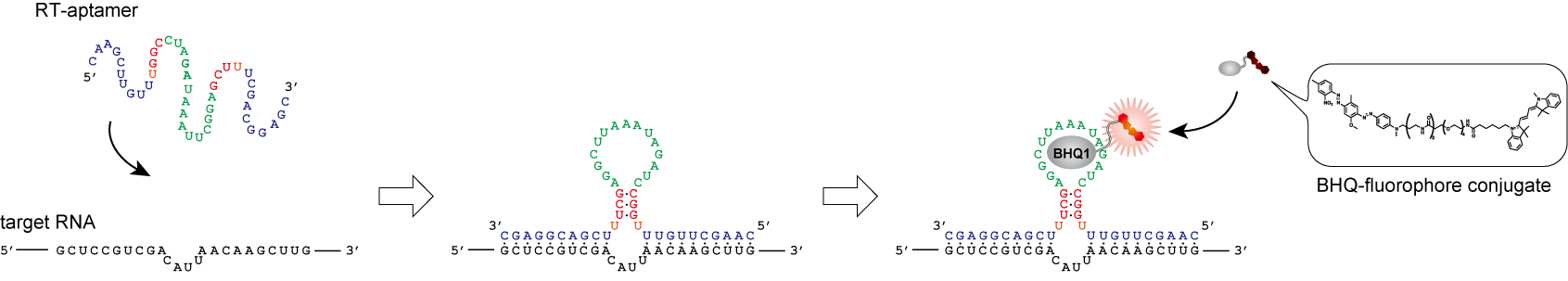
Live-cell imaging of endogenous mRNAs with a small molecule. †
Sato, S., Watanabe, M., Katsuda, Y., Murata, A., Wang, D. O., Uesugi M. †
Angew. Chem. Int. Ed. 54(6), 1855-1858 (2015)
https://doi.org/10.1002/anie.201410339
''
''
Synthetic molecules that protect cells from anoikis and their use in cell transplantation. †
Frisco-Cabanos, H.L., Watanabe, M., Okumura, N., Kusamori, K., Takemoto, N., Takaya, J., Sato, S., Yamazoe, S., Takakura, Y., Kinoshita, S., Nishikawa, M., Koizumi, N., Uesugi, M. †
Angew. Chem. Int. Ed. 53(42), 11208-11213 (2014)
https://doi.org/10.1002/anie.201405829
''
''
Selective elimination of human pluripotent stem cells by a marine natural product derivative. †
Kuo, T.F., Mao, D., Hirata, N., Khambu, B., Kimura, Y., Kawase, E., Shimogawa, H., Ojika, M., Nakatsuji, N., Ueda, K., Uesugi, M. †
J. Am. Chem. Soc. 136 (28), 9798-9801 (2014)
https://doi.org/10.1021/ja501795c
''
''
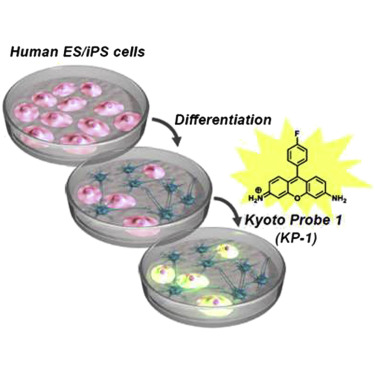
A chemical probe that labels human pluripotent stem cells. †
Hirata, N., Nakagawa, M., Fujibayashi, Y., Yamauchi, K., Murata, A., Minami, I., Tomioka, M., Kondo, T., Kuo, T.F., Endo, H., Inoue, H., Sato, S., Ando, S., Kawazoe, Y., Aiba, K., Nagata, K., Kawase, E., Chang, Y.T., Suemori, H., Eto, K., Nakauchi, H., Yamanaka, S., Nakatsuji, N., Ueda, K., Uesugi, M. †
Cell Rep 6., 1165–1174 (2014)
https://doi.org/10.1016/j.celrep.2014.02.006
''
''
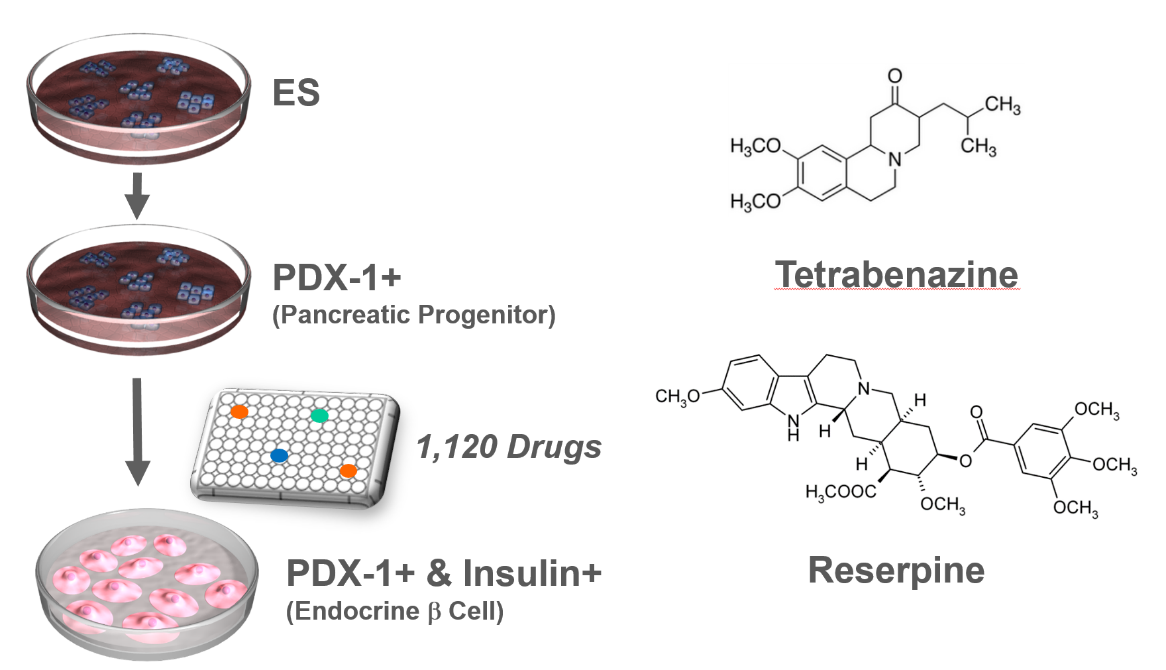
VMAT2 identified as a regulator of late-stage beta cell differentiation. †
Sakano, D., Shiraki, N., Kikawa, K., Yamazoe, T., Kataoka, M., Umeda, K., Araki, K., Mao, D., Matsumoto, S., Nakagata, N., Andersson, O., Stainier, D., Endo, F., Kume, K., Uesugi, M., Kume, S. †
Nat. Chem. Biol. 10, 141-148 (2014)
https://doi.org/10.1038/nchembio.1410
''
''
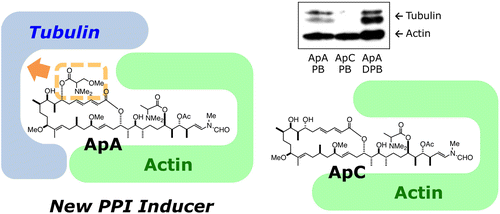
Inhibition of microtubule assembly by a complex of actin and antitumor macrolide aplyronine A. †
Kita, M., Hirayama, Y., Yoneda, K., Yamagishi, K., Chinen, T., Usui, T., Sumiya, E., Uesugi, M., Kigoshi, H. †
J. Am. Chem. Soc. 135 (48), 18089-18095 (2013)
https://doi.org/10.1021/ja406580w
''
''
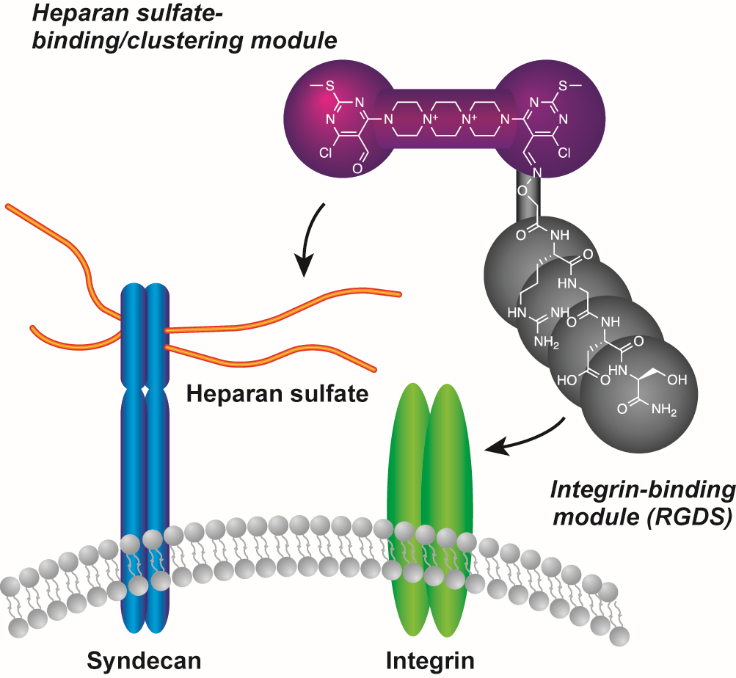
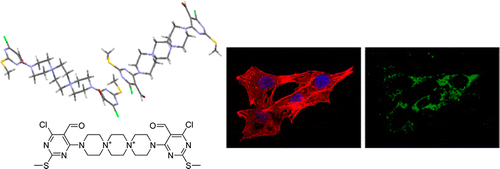
Small molecule-induced clustering of heparan sulfate promotes cell adhesion. †
Takemoto, N., Suehara, T., Frisco, H., Sato, S., Sezaki, T., Kusamori, K., Kawazoe, Y., Park, S., Yamazoe, S., Mizuhata, Y., Inoue, R., Miller, G., Hansen, S., Jayson, G., Gardiner, J., Kanaya, T., Tokitoh, N., Ueda, K., Takakura, Y., Kioka, N., Nishikawa, M., Uesugi, M. †
J. Am. Chem. Soc. 135 (30), 11032-11039 (2013)
https://doi.org/10.1021/ja4018682
''
''
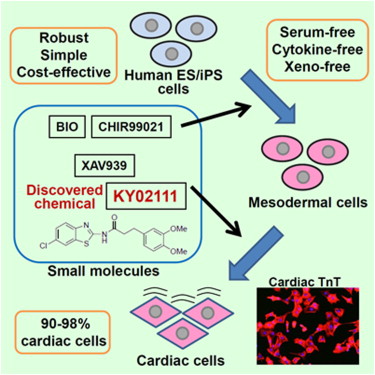
A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined cytokine- and xeno-free conditions. †
Minami, I., Yamada, K., Otsuji, T.G., Yamamoto, T., Shen, Y., Otsuka, S., Kadota, S., Morone, N., Barve, M., Asai, Y., Tenkova-Heuser, T., Heuser, J. E., Uesugi, M., Aiba, K., Nakatsuji, N. †
Cell Rep. (5), 1448-1460 (2012)
https://doi.org/10.1016/j.celrep.2012.09.015
''
''
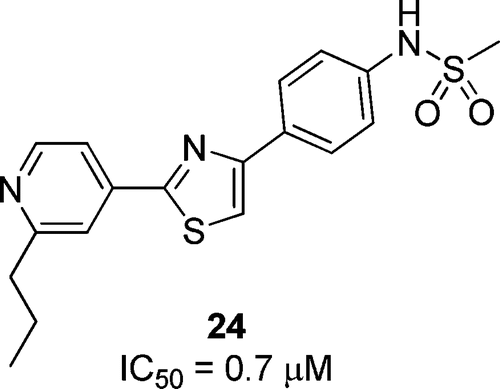
Synthesis and evaluation of diarylthiazole derivatives that inhibit activation of sterol regulatory element-binding proteins. †
Kamisuki, S., Shirakawa T., Kugimiya, A., Abu-Elheiga, L., Choo, H.-Y., Yamada, K., Shimogawa, H., Wakil, S. J., Uesugi, M. †
J. Med. Chem. 54(13), 4923-4927 (2011)
https://doi.org/10.1021/jm200304y
''
''
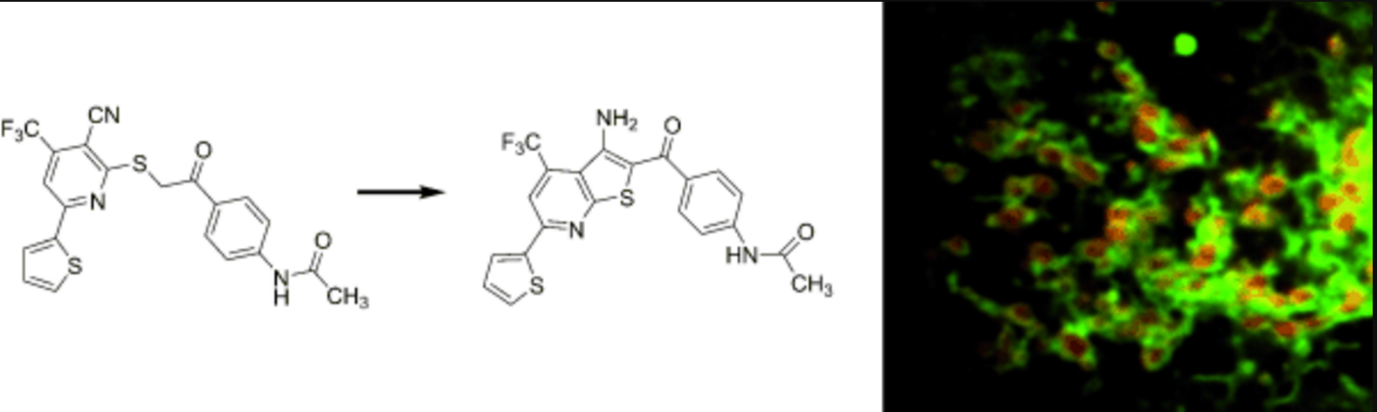
A mitochondrial surface-specific fluorescent probe activated by bioconversion. †
Kawazoe, Y., Shimogawa, H., Sato, A., Uesugi, M. †
Angew. Chem. Int. Ed. 50(24), 5478-5481 (2011)
https://doi.org/10.1002/anie.201100935
''
''
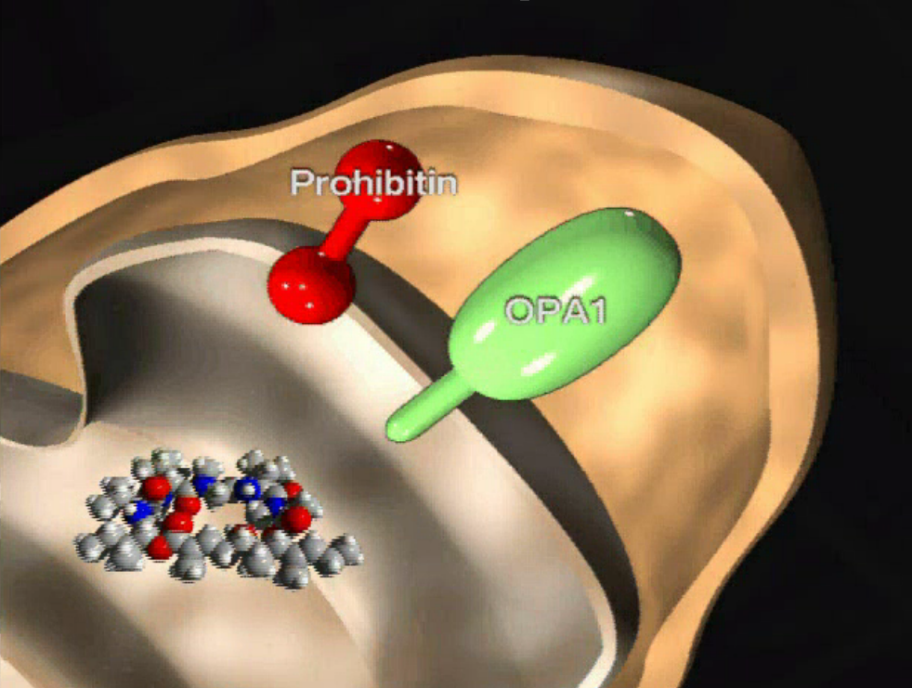
Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. †
Sato, S. Murata, A., Orihara, T., Shirakawa, T., Suenaga, K., Kigoshi, H., Uesugi, M. †
Chem. Biol. 18 (1), 131-139 (2011)
https://doi.org/10.1016/j.chembiol.2010.10.017
''
''
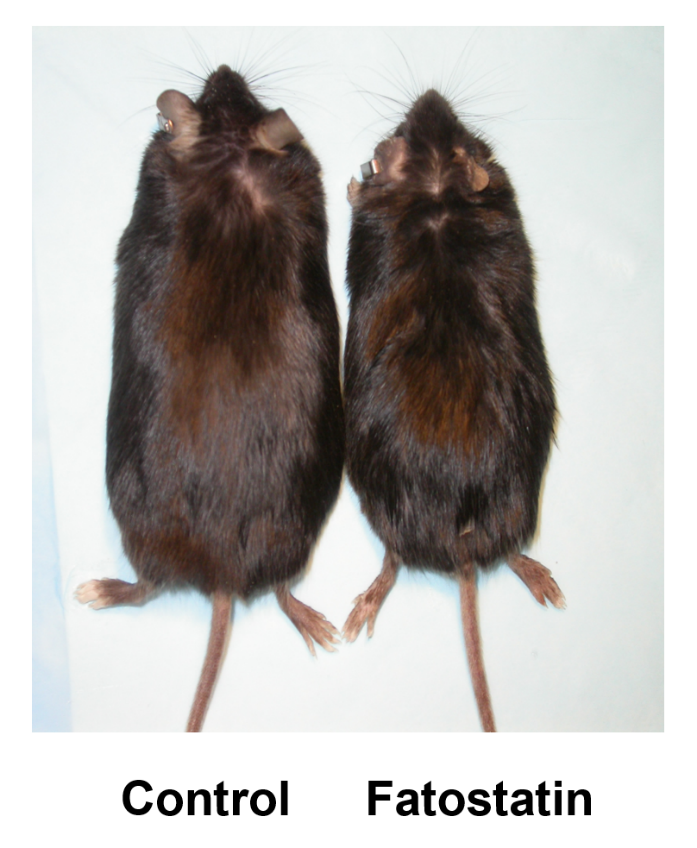
A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. †
Kamisuki, S., Mao, Q., Abu-Eliheiga, L., Gu, Z., Kugimiya, A., Kwon, Y., Shinohara, T., Kawazoe, Y., Sato, S. Asakura, K., Choo, H., Sakai, J., Wakil, SJ., Uesugi, M. †
Chem. Biol. 16 (8), 882-892 (2009)
https://doi.org/10.1016/j.chembiol.2009.07.007
''
''
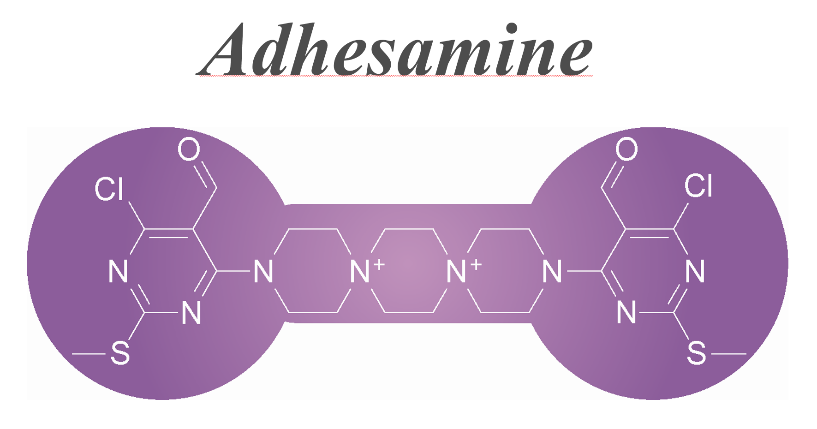
A dumbbell-shaped small molecule that promotes cell adhesion and growth. †
Yamazoe, S., Shimogawa, H., Sato, S., Esko, J. D., Uesugi, M. †
Chem. Biol. 16 (7), 773-782 (2009)
https://doi.org/10.1016/j.chembiol.2009.06.008
''
''
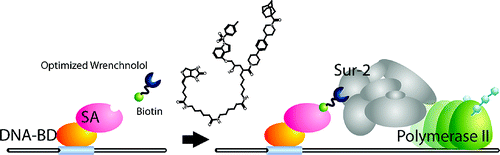
Wrenchnolol derivative optimized for gene activation in cells. †
Jung, D., Shimogawa, H., Kwon, Y., Mao, Q., Sato, S., Kamisuki, S., Kigoshi, H., Uesugi, M. †
J. Am. Chem. Soc. 131(13), 4774-4782 (2009)
https://doi.org/10.1021/ja900669k
''
''
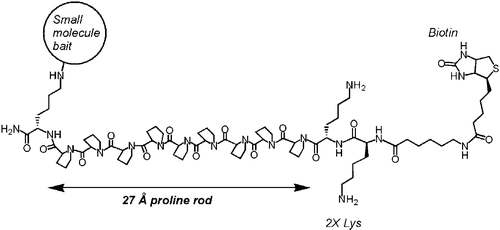
Polyproline-rod approach to isolating protein targets of bioactive small molecules: isolation of a new target of indomethacin. †
Sato, S., Kwon, Y., Kamisuki, S., Srivastava, N., Mao, Q., Kawazoe, Y., Uesugi, M. †
J. Am. Chem. Soc. 129(4), 873-880 (2007)
https://doi.org/10.1021/ja0655643
''
''
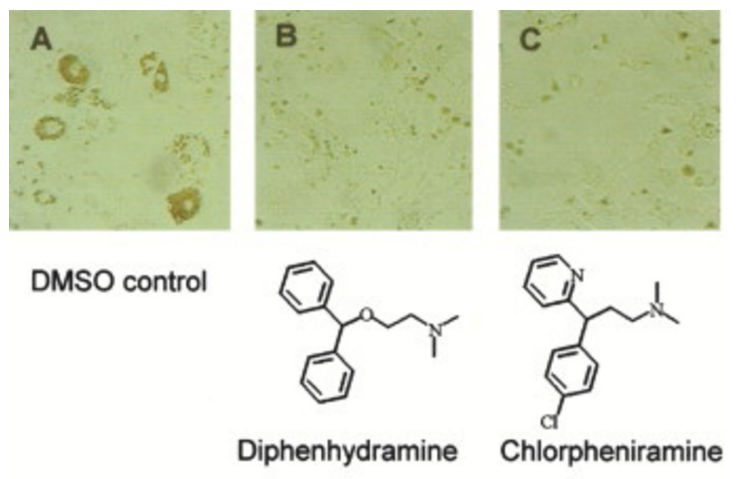
Chemical genetic identification of histamine receptor H1 as a stimulator of insulin-induced adipogenesis. †
Kawazoe, Y., Tanaka, H., Uesugi, M. †
Chem. Biol. 11, 907-913 (2004)
https://doi.org/10.1016/j.chembiol.2004.04.017
''
''
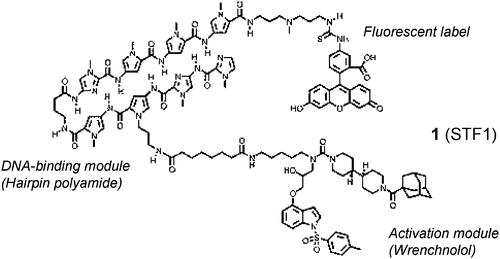
Small molecule transcription factor mimic. †
Kwon,Y., Arndt, H., Mao, Q., Choi, Y., Kawazoe, Y., Dervan, P. B., Uesugi, M. †
J. Am. Chem. Soc. 126, 15940-15941 (2004)
https://doi.org/10.1021/ja0445140
''
''
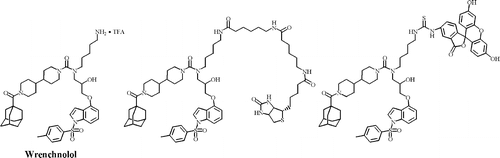
A wrench-shaped synthetic molecule that modulates a transcription factor-coactivator interaction. †
Shimogawa, H., Kwon, Y., Mao, Q., Kawazoe, Y., Choi, Y., Asada, S., Kigoshi, H., & Uesugi, M. †
J. Am. Chem. Soc. 126, 3461-3471 (2004)
https://doi.org/10.1021/ja038855+
''
''
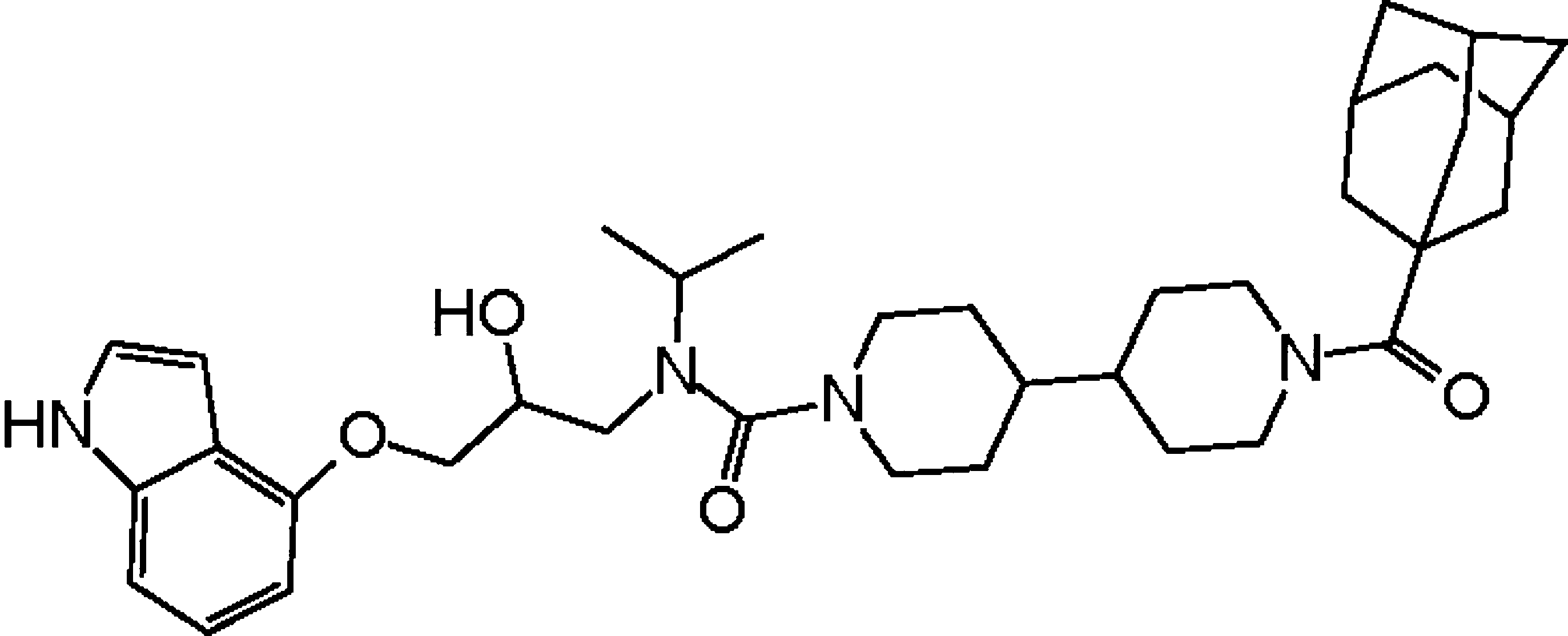
A gene-expression inhibitor that targets an alpha-helix-mediated protein interaction. †
Asada, S., Choi, Y., & Uesugi, M. †
J. Am. Chem. Soc. 125, 4992-4993 (2003)
https://doi.org/10.1021/ja0292703
''
''
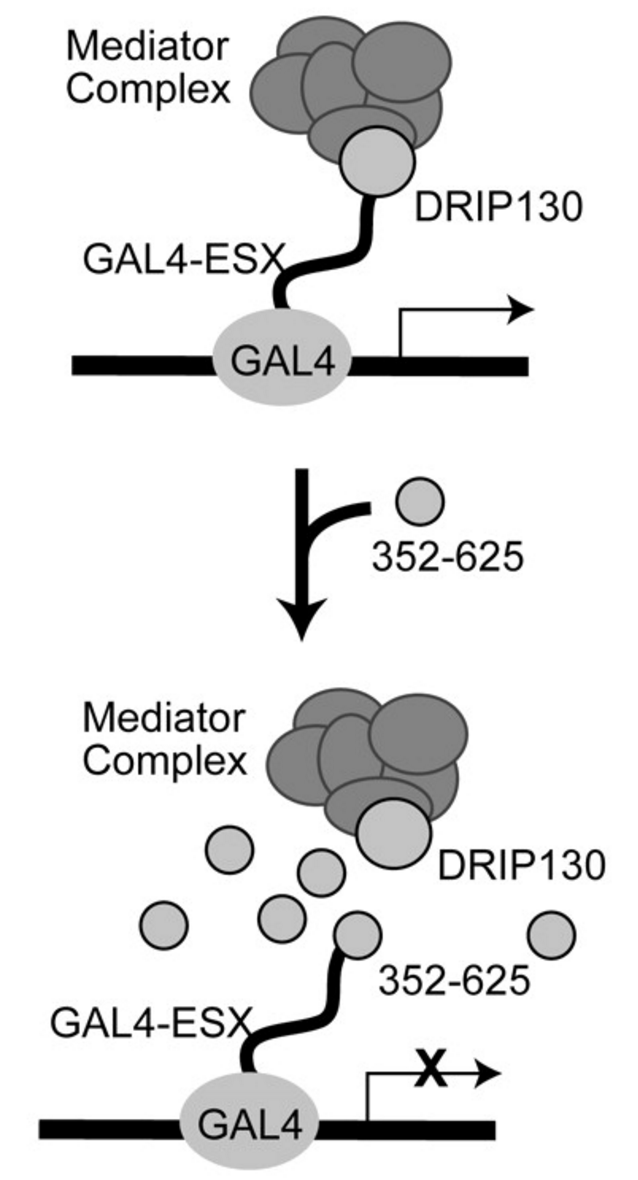
External control of Her2 expression and cancer cell growth by targeting a Ras-linked coactivator. †
Asada, S., Choi, Y., Yamada, M., Wang, S., Hung, M., Qin, J., & Uesugi, M. †
Proc. Natl. Acad. Sci. USA 99, 12747-12752 (2002)
https://doi.org/10.1073/pnas.202162199
''
''
Divergent hTAF(II)31-binding motifs hidden in activation domains. †
Choi, Y., Asada, S., & Uesugi, M. †
J. Biol. Chem. 275, 15912-15916 (2000)
https://doi.org/10.1074/jbc.275.21.15912

''
''
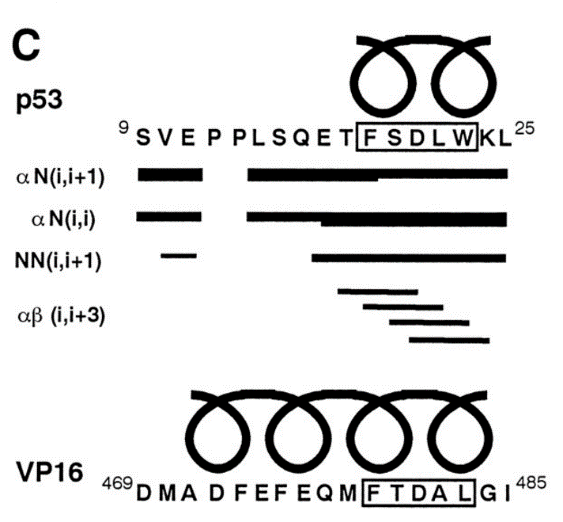
The alpha helical FXXphiphi motif in p53: TAF interaction and discrimination by MDM2. †
Uesugi, M. & Verdine, G. L. †
Proc. Natl. Acad. Sci. USA 96, 14801-14806 (1999)
https://doi.org/10.1073/pnas.96.26.14801
''
''
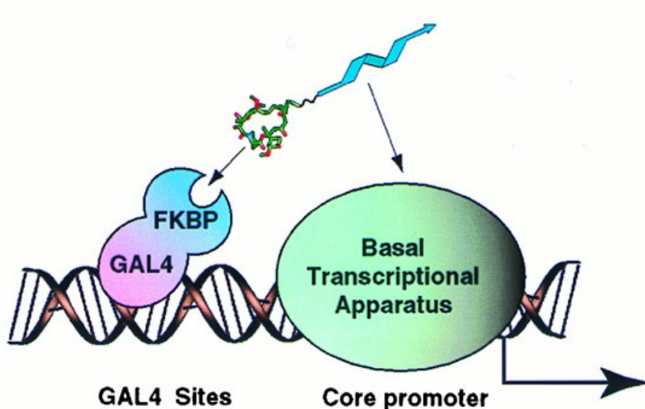
A non-natural transcriptional coactivator. †
Nyanguile, O., Uesugi, M., Austin, D. J., & Verdine, G. L. †
Proc. Natl. Acad. Sci. USA 94, 13402-13406 (1997)
https://doi.org/10.1073/pnas.94.25.13402
''
''
Induced alpha helix in the VP16 activation domain upon binding to a human TAF. †
Uesugi, M., Nyanguile, O., Lu, H., Levine, A. J., & Verdine, G. L. †
Science 277, 1310-1313 (1997)
https://doi.org/10.1126/science.277.5330.1310